|
Increases in Diabetes: Who is at Risk?
The prevalence of type 2 diabetes has increased dramatically. According to the Centers for Disease Control (CDC), 10% of the US population has diabetes today. By the year 2050, the CDC projects that 21% to 33% will be diagnosed with diabetes. This will lead to a 2- to 4-fold increase in health-care costs, or approximately $171 billion per year.
With these alarming projections, it becomes increasingly important to define the stages of progression to diabetes and focus on treatments that prevent the progression to diabetes. According to the CDC, "Progression to diabetes among those with prediabetes is not inevitable. Studies have shown that people with prediabetes … can prevent or delay diabetes."1
Only 38% of adults at risk of developing diabetes are obese. How do you indentify the other 62%? Doctors assume that they can accurately predict patient risk for diabetes based on obesity. As a result, many question the need for a test designed to assess risk of diabetes. Simply looking at a patient or calculating BMI to determine who should be tested for diabetes is a common misconception. In fact 32% of obese patients are not insulin-resistant and 25% of healthy-weight patients have insulin resistance.2,3
Progression to Diabetes
No single biomarker provides the complete view into the current status of a patient's progression to diabetes. The PreD Guide, a diagnostic tool developed at Genova Diagnostics, integrates clinical markers for blood sugar regulation (fasting glucose and HbA1c), key biomarkers of metabolism (adiponectin, insulin, and proinsulin), and inflammatory biomarkers to provide insight into a patient's progression toward diabetes and underlying conditions, giving more guidance for therapeutic interventions to reverse the progression to diabetes.
Key Biomarkers and the Stages of Prediabetes
Adiponectin is an adipocyte-derived protein that plays an important role in glucose and lipid metabolism.4 Besides increasing hepatic and peripheral insulin sensitivity, moderating fat tissue growth, and decreasing lipid and glucose production in the liver, adiponectin lowers blood pressure and protects against atherosclerosis by suppressing vascular inflammation.5 Adiponectin levels tend to be inversely associated with body mass index (BMI), insulin resistance, triglycerides, blood pressure, C-reactive protein, and risk of type 2 DM.6 Normal levels of adiponectin protect against insulin resistance, obesity, and cardiovascular disease (CVD).7
Insulin is a hormone produced in the beta cells of the pancreas upon stimulation by glucose. Insulin induces liver, adipose, and muscle cells to take up glucose from the blood. Insulin also helps store glucose in the liver as glycogen, suppresses hepatic glucose synthesis, and inhibits the breakdown of fat.8 Insulin secretion consists of two phases: an initial rapid release of insulin in response to glucose, followed by a more sustained secretion lasting several hours until glucose has normalized.9 Although insulin resistance is often reflected by an elevated fasting insulin level, in some individuals this is only apparent in post-glucose-load measurements.10
Proinsulin is the precursor of insulin and is made within the beta cells of the pancreas. Because cleavage of proinsulin to make insulin takes place within the beta cells, higher levels of circulating proinsulin indicate advancing beta cell dysfunction and increased risk or presence of diabetes.11 Proinsulin also serves as a marker of insulin resistance, since chronic insulin resistance leads to compensatory production of insulin. As the cells become exhausted, cleavage capacity of proinsulin declines, and circulating levels increase.11 Proinsulin is the precursor of insulin and is made within the beta cells of the pancreas.
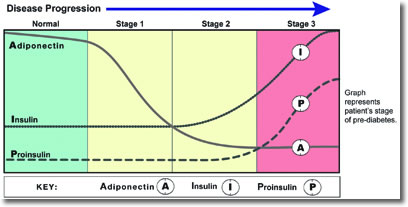
The interplay of these markers is illustrated across the stages of prediabetes (see Figure 1).
Figure 1
Stage 1: Early Insulin Resistance
Stage 1 of metabolic dysglycemia represents early insulin resistance, with adequate pancreatic beta-cell compensation to maintain normal glucose. Insulin level may be normal or high. Adiponectin, which provides protection against insulin resistance, diabetes, and cardiovascular disease declines. Dyslipidemia may or may not be present, including elevated triglycerides and LDL-C, and/or low HDL-C. At this stage, dietary and lifestyle measures are usually adequate for improving insulin sensitivity and preventing progression to Stage 2.
Stage 2: Elevated Insulin
Stage 2 represents impaired glucose tolerance, usually due to combination of insulin resistance and early pancreatic beta-cell impairment. In most cases of insulin resistance, compensatory increased insulin secretion is sufficient to prevent hyperglycemia. However, in combination with beta-cell dysfunction, hyperglycemia can develop.12 Adiponectin is usually low, and glucose and/or HbA1c are elevated, although not yet to a diabetic level. Insulin is usually elevated. Dyslipidemia may or may not be present, including elevated triglycerides and LDL-C, and/or low HDL-C. At this stage, diet and lifestyle measures, along with supplementation can help improve insulin sensitivity, restore proper glucose regulation, and prevent progression to Stage 3.
Stage 3: Beta-Cell Dysfunction
Stage 3 represents the development of diabetes, with insulin resistance and progressive pancreatic beta-cell impairment. Beta-cell dysfunction can result from glucose toxicity, inflammatory cytokines, oxidative stress, and/or lipotoxicity in the presence of excess glucose.12,13 Glucose and HbA1c are significantly elevated, and insulin may or may not be elevated, depending on beta-cell capacity to produce adequate insulin. Sequential measurements can help reveal the degree of beta-cell dysfunction; declining insulin along with increasing proinsulin signifies late-stage impairment. The most important therapeutic goal at this stage is to normalize and maintain normal blood glucose levels.14 At this stage, a comprehensive approach is essential, including diet and lifestyle measures, supplementation, and targeted pharmaceuticals, based on the degree of beta-cell impairment.
Role of Low-Grade Inflammation
There is an evolving body of evidence to support that inflammation mediates the pathogenesis of insulin resistance, type 2 diabetes, and cardiovascular disease. High concentrations of circulating cytokines, C-reactive protein (CRP), interleukin-6 (IL-6), interleukin-8 (IL-8), plasminogen-activating inhibitor (PAI-1), and tumor necrosis factor alpha (TNF-α) have been associated with the development of type 2 diabetes.15,16
Adipose tissue (especially visceral) is an active endocrine organ that produces numerous "adipokines," including inflammatory cytokines. These inflammatory mediators are central to the pathophysiology of obesity and its systemic effects, including insulin resistance, diabetes, atherosclerosis, and fatty liver.17 They also encourage further deposition of visceral fat, thus creating a vicious cycle. Inflammation has been seen to directly correlate with risk of type 2 diabetes.18
A better understanding of the role that chronic inflammation plays in promoting the development and progression of various chronic diseases may provide a common mechanistic causality. The correlation has been well established; however, the specific mechanism by which inflammation causes insulin resistance is still under investigation.
Pattern recognition receptors (PRRs) mediate chronic inflammation; PRRs include both Toll-like receptors (TLRs) and nucleotide-binding oligomerization domain proteins (NODs) that detect gut bacterial flora, assess phytochemicals, and modulate the innate immune response. Understanding of this process provides interesting insight into inflammation's effect on insulin sensitivity and possible areas of treatment intervention. For example, TLR4 activates IkappaB kinase β (IKKβ), which impedes the insulin receptor signaling, as well as increasing production of nuclear factor κB's (NF-κB) expression of inflammatory cytokines. Dietary factors that inhibit TLR activation, such as curcurmin and sulforaphane, can thus improve insulin sensitivity.19
The use of multiple inflammatory markers provides greater insight into the effects of inflammation. One marker for inflammation does not provide a full clinical picture.
Inflammatory Markers
High-sensitivity C-Reactive Protein (hs-CRP) is an acute-phase response protein produced within the liver and atherosclerotic arteries. CRP is an independent risk marker for coronary events in individuals without overt hyperlipidemia, thus adding prognostic information to cardiovascular risk assessment.20 Although adipose tissue is an important determinant of a chronic, low-level inflammatory state as reflected by levels of hs-CRP and inflammatory cytokines,21 insulin resistance rather than obesity appears to be the major determinant of hs-CRP levels.22 Elevated hs-CRP predicts the development of type 2 diabetes.22,23
Tumor necrosis factor-alpha (TNF-α) and interleukins such as IL-6 and IL-8 are immunoregulatory "cytokines" that are produced in a wide variety of tissues in the body. In high amounts (such as from visceral fat),24 these cytokines mediate the acute phase response and contribute to chronic inflammation. CRP levels are largely determined by inflammatory cytokines, especially IL-6.25
Levels of IL-6, IL-8 and/or TNF-α are generally increased in overweight and obese individuals,15 and are known to induce insulin resistance, although this can also occur independent of obesity.26 Elevated levels of IL-6, IL-8 and/or TNF-α are independent predictors of the development of type 2 diabetes.27-29
Plasminogen activating inhibitor-1 (PAI-1) is the primary inhibitor of plasminogen activation. Plasminogen is an acute phase protein and precursor to plasmin, which digests fibrin, the essential portion of a blood clot. Elevated levels of PAI-1 thus predispose to clot formation by inhibiting fibrinolytic activity. PAI-1 is mainly produced by the endothelium, but is also secreted by fat tissue, especially abdominal, which has been found to express 5-fold more PAI-1 compared with subcutaneous adipose in obese individuals.30 Evidence supports the association of PAI-1 with the development of type 2 diabetes independent of body fat and insulin resistance, suggesting that it maybe an early risk marker.16
The activation of inflammation is a heterogeneous process, so one must look at an array of biomarkers to assess risk. The Pre-D Guide is unique in providing an average inflammation score calculated from these markers of inflammation to determine the relative risk, as no single marker of inflammation provides the whole picture. This score helps distinguish minimal, moderate, and severe inflammation.
Reversibility, Personalized Treatment
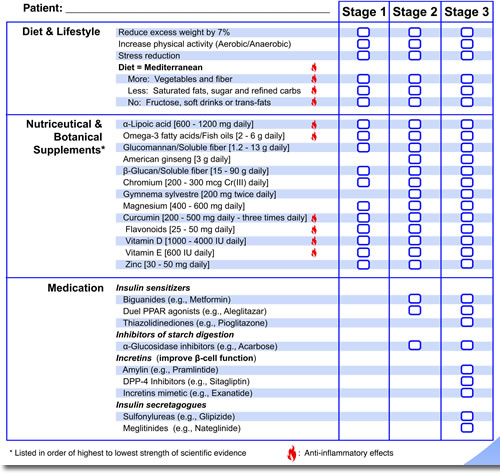
Studies show that people with prediabetes can prevent or delay the development of type 2 diabetes by up to 58%.31 Diet and exercise can make dramatic improvements in weight loss and increasing insulin sensitivity. More effective treatments for later stages of prediabetes in addition to diet and exercise include the use of nutriceuticals, botanicals, and medications. The Pre-D Guide provides stage-specific therapeutic interventions (see figure 2).
Figure 2
Summary
Diagnostic tools that move beyond BMI to identify patients at risk for developing diabetes are clinically important to effectively address the epidemic of diabetes.
Integrating traditional markers of blood glucose regulation with inflammatory and metabolic biomarkers, to pinpoint a patient's place within the progression to diabetes, allows for stage-specific treatments to effectively prevent the development of type 2 diabetes.

 Patrick Hanaway, M.D., is a board-certified family physician and past president of the American Board of Integrative Holistic Medicine. As Chief Medical Officer of Genova Diagnostics, Dr. Hanaway's concentration is on the clinical implementation of applied nutritional biochemistry, with an emphasis on digestive disease and wellness. He is responsible for all clinical research activities and Genova Diagnostics' health education program. Dr. Hanaway reviews product quality initiatives, provides medical consultations for clients, and directs the strategic development of new and innovative diagnostic tests. He is a graduate of the University of Wisconsin and the Washington University School of Medicine with residency training at the University of New Mexico. Patrick Hanaway, M.D., is a board-certified family physician and past president of the American Board of Integrative Holistic Medicine. As Chief Medical Officer of Genova Diagnostics, Dr. Hanaway's concentration is on the clinical implementation of applied nutritional biochemistry, with an emphasis on digestive disease and wellness. He is responsible for all clinical research activities and Genova Diagnostics' health education program. Dr. Hanaway reviews product quality initiatives, provides medical consultations for clients, and directs the strategic development of new and innovative diagnostic tests. He is a graduate of the University of Wisconsin and the Washington University School of Medicine with residency training at the University of New Mexico.
 Teresa P. McBride, N.D., received her doctorate of Naturopathic medicine from the Southwest College of Naturopathic Medicine in Tempe, Arizona. She is currently part of the medical education team at Genova Diagnostics helping to support practitioners with the clinical application of Genova Diagnostic laboratory tests in the areas of nutritional biochemistry, gastrointestinal health, endocrinology and genomics. Dr. McBride was in clinical practice with a focus on primary care prior to joining the staff at Genova Diagnostics. Dr. McBride has done research and consulting for the natural foods industry in the areas of diet and nutrition. Teresa P. McBride, N.D., received her doctorate of Naturopathic medicine from the Southwest College of Naturopathic Medicine in Tempe, Arizona. She is currently part of the medical education team at Genova Diagnostics helping to support practitioners with the clinical application of Genova Diagnostic laboratory tests in the areas of nutritional biochemistry, gastrointestinal health, endocrinology and genomics. Dr. McBride was in clinical practice with a focus on primary care prior to joining the staff at Genova Diagnostics. Dr. McBride has done research and consulting for the natural foods industry in the areas of diet and nutrition.

Notes
1. Centers for Disease Control and Prevention (CDC). National diabetes fact sheet, 2007 [online document]. http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2007.pdf.
2. Wildman R et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlated for 2 phenotypes among the US population (NHANES 1999–2004). Arch Intern Med. 2008;168(15):1617–1624.
3. CDC. Prevalence and trends data [Web page]. http://apps.nccd.cdc.gov/brfss/display.asp?cat=OB&yr=2009&qkey=4409&state=US.
4. Chandran M, Phillips SA, Ciaraldi T, Henry RR. Adiponectin: more than just another fat cell hormone? Diabetes Care. 2003 Aug;26(8):2442–50
5. Stern N, Osher E, Greenman Y. Hypoadiponectinemia as a marker of adipocyte dysfunction – Part 1: the biology of adiponectin. J Cardiometab Syndr. 2007 Summer;2(3):174–182.
6. Pischon T, Girman CJ, Hotamisiligil GS, et al. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA. 2004 Apr 14;291(14):1730–1737.
7. Lawlor DA, Davey Smith G, Ebrahim S, et al. Plasma adiponectin levels are associated with insulin resistance, but do not predict future risk of coronary heart disease in women. J Clin Endocrinol Metab. 2005 Oct;90(10):5677–5683.
8. Kendall DM, Harmel AP. The metabolic syndrome, type 2 diabetes, and cardiovascular disease: understanding the role of insulin resistance. Am J Manag Care. 2002 Dec;8(20Suppl): S635–S653.
9. Kahn SE, Montogomery B, Howell W, et al. Importance of early phase insulin secretion to intravenous glucose tolerance in subjects with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2001 Dec;86(12):5824–5829.
10. Leiter LA, Ceriello A, Davidson JA, et al. Postprandial glucose regulation: new data and new implications. Clin Ther. 2005;27 Suppl B:S42–56.
11. Pfutzner A, Weber MM, Forst T. A biomarker concept for assessment of insulin resistance, beta-cell function and chronic systemic inflammation in type2 diabetes mellitus. Clin Lab. 2008;54(11–12):485–490.
12. Wajchenberg BL. Beta-cell failure in diabetes and preservation by clinical treatment. Endocr Rev. 2007 Apr;28(2):187–218.
13. Maiese K, Chong ZZ, Shang YC. Mechanistic insights into diabetes mellitus and oxidative stress. Cur Med Chem. 2007;14(16):1729–1738.
14. Hanefeld M, Temelkova-Kurtshiev T. Control of post-prandial hyperglycemia – an essential part of good diabetes treatment and prevention of cardiovascular complications. Nutr Metab Cardiovasc Dis. 2002 Apr;12(2):98–107.
15. Brunn JM, Verdich C, Toubro S, et al. Association between measures of insulin sensitivity and circulating levels of interleukin-8, interleukin-6 and tumor necrosis factor-alpha. Effect of weight loss in obese men. Eur J Endocrinol. 2003 May;148(5):535–542.
16. Fiesta A, D'Agostino R Jr, Tracy RP, et al. Elevated levels of acute-phase proteins and plasminogen activator inhibitor-1 predict the development of type 2 diabetes. Diabetes. 2002 Apr;51(4):1131–1137.
17. De Ferranti S, Mozaffarian D. The perfect storm: obesity, adipocyte dysfunction and metabolic consequences. Clin Chem. 2008 Jun;54(6):945–955.
18. Liu S, Tinker L, Song Y et al. A prospective study of inflammatory cytokines and diabetes mellitus in a multiethnic cohort of postmenopausal women. Arch Intern Med. 2007 Aug 13–27;167(15):1676–1685
19. Zhao L, Lee J, Hwang D. Inhibition of pattern recognition receptor-mediated inflammation by bioactive phytochemicals. Nutr Rev. 2011 Jun;69(6):310–320.
20. Ridker PM. High-sensitivity C-reactive protein and cardiovascular risk: rational for screening and primary prevention. Am J Cardiol. 2003 Aug 21;92(4B):17K–22K.
21. Yudkin JS, Stehouwer CD, Emeeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999 Apr;19(4):972–978.
22. Festa A, Hanley AJ, Tracy RP, et al. Inflammation in the prediabetic state is related to increased insulin resistance rather than decreased insulin secretion. Circulation. 2003 Oct 14;108(15):1822–1830.
23. Freeman DJ, Norrie J, Caslake MJ, et al. C-reactive protein is an independent predictor of risk for the development of diabetes in the West of Scotland Coronary Prevention Study. Diabetes. 2002 May;51(5):1596–1600.
24. Wellen KE, Hotamisiligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005 May;115(5):1111–1119.
25. Yudkin JS, Stehouwer CD, Emeiss JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999 Apr;19(4):972–978.
26. Pradhan AD, Manson JE, Rifai N, et al. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001 Jul 18;286(3):327–334.
27. Wannamethee SG, Lowe GD, Rumley A, et al. Adipokines and risk of type 2 diabetes in older men. Diabetes Care. 2007 May;30(5):1200–1205.
28. Herder C, Haastert B, Muller-Scholze S, et al. Association of systemic chemokine concentrations with impaired glucose tolerance and type 2 diabetes; results from the Cooperative Health Research in the Region of Augsburg Survey S4 (KORA S4). Diabetes. 2005 Dec;54 Suppl 2:S11–S17.
29. Hu FB, Meigs JB, Li TY, et al. Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes. 2004 Mar;53(3):693–700.
30. Alessi MC, Juhan-Vague I. PAI-1 and the metabolic syndrome: links, causes, and consequences. Arterioscler Thromb Vasc Biol. 2006 Oct;26(10):2200–2207.
31. American Diabetes Association. Prediabetes FAQ [Web page]. http://www.diabetes.org/diabetes-basics/prevention/pre-diabetes/
pre-diabetes-faqs.html. (One link, two lines.)


|
|



![]()
![]()
![]()



 Patrick Hanaway, M.D., is a board-certified family physician and past president of the American Board of Integrative Holistic Medicine. As Chief Medical Officer of Genova Diagnostics, Dr. Hanaway's concentration is on the clinical implementation of applied nutritional biochemistry, with an emphasis on digestive disease and wellness. He is responsible for all clinical research activities and Genova Diagnostics' health education program. Dr. Hanaway reviews product quality initiatives, provides medical consultations for clients, and directs the strategic development of new and innovative diagnostic tests. He is a graduate of the University of Wisconsin and the Washington University School of Medicine with residency training at the University of New Mexico.
Patrick Hanaway, M.D., is a board-certified family physician and past president of the American Board of Integrative Holistic Medicine. As Chief Medical Officer of Genova Diagnostics, Dr. Hanaway's concentration is on the clinical implementation of applied nutritional biochemistry, with an emphasis on digestive disease and wellness. He is responsible for all clinical research activities and Genova Diagnostics' health education program. Dr. Hanaway reviews product quality initiatives, provides medical consultations for clients, and directs the strategic development of new and innovative diagnostic tests. He is a graduate of the University of Wisconsin and the Washington University School of Medicine with residency training at the University of New Mexico. Teresa P. McBride, N.D., received her doctorate of Naturopathic medicine from the Southwest College of Naturopathic Medicine in Tempe, Arizona. She is currently part of the medical education team at Genova Diagnostics helping to support practitioners with the clinical application of Genova Diagnostic laboratory tests in the areas of nutritional biochemistry, gastrointestinal health, endocrinology and genomics. Dr. McBride was in clinical practice with a focus on primary care prior to joining the staff at Genova Diagnostics. Dr. McBride has done research and consulting for the natural foods industry in the areas of diet and nutrition.
Teresa P. McBride, N.D., received her doctorate of Naturopathic medicine from the Southwest College of Naturopathic Medicine in Tempe, Arizona. She is currently part of the medical education team at Genova Diagnostics helping to support practitioners with the clinical application of Genova Diagnostic laboratory tests in the areas of nutritional biochemistry, gastrointestinal health, endocrinology and genomics. Dr. McBride was in clinical practice with a focus on primary care prior to joining the staff at Genova Diagnostics. Dr. McBride has done research and consulting for the natural foods industry in the areas of diet and nutrition.