|
In the past, clinical laboratory analysis of circulating body fluids has been done using serum, which necessitates a venipuncture. Since the latter part of the 20th century, saliva has been used increasingly for this purpose. Saliva has proven to be a readily available and accurate substitute for serum to identify markers of endocrine, immunologic, inflammatory, infectious, and other diagnostic problems. Recently, a consortium of three research groups categorized 1116 proteins secreted in saliva, and stated that "[such] studies can be used to translate blood based clinical laboratory tests into a format that utilizes saliva.1 Saliva has been categorized "as a valuable biofluid … with the potential to extract more data than is possible currently with other diagnostic methods.2 A noninclusive list of current saliva testing is presented in Table 1.
Table 1: Selected Conditions Detected through Salivary Testing
- Adrenal conditions (e.g., Addison's/Cushing's)
- Neoplasms (e.g., benign and metastic)
- Altered female hormone states (e.g., PCOS, menopause, infertility)
- Infectious conditions (e.g., HIV, hepatitis, Helicobacter pylon)
- Altered male hormone states (e.g., hypogonadism, hyperestrogenism)
- Allergic states (e.g., asthma, mold, food)
- Metabolic disturbances (e.g., insulin resistance, diabetes)
- Drugs (e.g., marijuana, cocaine, alcohol)
Background
Laboratory testing for hormones using saliva was verified in the 1980s and 1990s, and it is becoming increasingly more utilized clinically, particularly for endocrinologic and immunologic assays. The slow pace of its acceptance appears to have been the result of a variety of factors: most clinicians are unfamiliar with the benefits and availability of salivary testing; the majority of laboratories have made significant investments in blood analysis systems and equipment; there is an innate reluctance to waiver from serum testing, which has been the basis for most research and clinical decision-making; and, until recently, there has been little criticism of the existing serum testing.
Hormone testing has been carried out traditionally by assaying levels in serum. This type of testing is beleaguered by nature's form of transport of hormones in the blood: over 90% of the hormones in blood are bound to proteins (binding globulins) and are inactive; the small percentage remaining are considered "free, or "available, to traverse the capillary membranes into the tissues and have their effect on target cells. There are major problems, however, inherent in serum hormone testing: there is no assurance that the "available hormone calculated actually reaches the tissue fluid to affect its target cells; and there exists a constant interchange between "bound and free hormones in the blood, a dynamic that calls into question whether any measured serum free values actually are meaningful.
These shortcomings have been recognized by the Endocrine Society for testosterone, the hormone with the lowest free fraction (<3%). In 2008, the Endocrine Society issued a position statement stating that "the manner in which most [serum] assays for TT [total testosterone] and FT [free testosterone] are currently performed is decidedly unsatisfactory.3 Recognizing that "important discrepancies and inconsistencies in measurements are widespread, in 2010 the Endocrine Society paired with the Centers for Disease Control (CDC), other clinical societies, and commercial laboratories to endorse "accuracy-based testing of testosterone and calibration of all methods traceable to a single high-level reference material.4 This process is expected to take several years overall. These methods are being developed by the CDC using mass spectrometry and offered to all interested parties. At the time of this writing, only five laboratories appear to have been so certified; the four in the US use expensive mass spectroscopy. In light of this issue, the editors of the Journal of Clinical Endocrinology and Metabolism raised the question as to whether "all of our other routinely employed [serum] hormonal assays provide truly accurate and precise measurements.5 Faced with major imperfections in and doubts about serum hormone testing, clinicians are seeking other forms of hormone testing that don't have protein-binding and capillary-transfer problems. A widespread and accurate form of hormone testing, one that is not subject to these limitations, utilizes saliva.
Saliva Testing Accuracy
Salivary hormone testing is suitable because it does not entail any of the problems inherent to serum testing; it measures levels in the tissue fluid, where there are no binding proteins. The 2007 edition of Greenspan's textbook Basic and Clinical Endocrinology states that "salivary cortisol reflects 'free' cortisol, and saliva testing directly measures active [emphasis added] steroid hormone ('free' hormone).6
Salivary hormone testing was fully verified in the 1980s, at which time any problems with laboratory methods, specimen collection, and specimen transport were settled.7-11 In 1994, it was reported that more than 2500 clinical and research articles dealing with the accuracy and application of salivary diagnostic tests had been published.12 These papers emphasized that close correlation exists between salivary testing (free fraction) and the free fraction of the serum; needless to say, no correlation was present with the serum protein bound fraction. Since then, several review articles have further verified and vindicated salivary hormone analysis.13-19
Chronobiologic Testing
Not only is saliva testing more accurate, inasmuch as it measures only the active free hormone, but it also allows chronobiologic testing, which is not feasible with serum testing with its attendant venipunctures. Championed by Franz Halberg of the University of Minnesota, chronobiology is defined as the field of science that examines periodic (cyclic) phenomena in living organisms. One principal application of chronobiology is circadian cortisol output assessment. Chronobiologic assessment of the adrenal glands' diurnal/circadian rhythm using multiple cortisol samplings throughout the day can only be accomplished, with absolute accuracy, via nonstressful saliva testing.
Adrenal Cortisol Testing
In 2008, the Endocrine Society published guidelines for the diagnosis of Cushing's syndrome. One of the four recommended initial screening tests is late-night salivary cortisol measurement (on two consecutive days).20 Ample references are provided to show that "free cortisol in the blood is in equilibrium with cortisol in the saliva; that "the concentration of salivary cortisol does not appear to be affected by a change in the rate of salivary production; and that "an increase in blood cortisol is reflected by a change in the salivary cortisol concentration within a few minutes.20 As noted, the accuracy of a single salivary cortisol assay has been attested to and validated formally by the Endocrine Society. It logically follows that cortisol samples collected at other times of the day equally must be valid, using proper technology. Such technology can be by either (costly) mass spectrometry or (economical) ELISA techniques; both "yield a 92%-100% sensitivity and a 93%-100% specificity.20
The first salivary test panel offered by Diagnos-Techs in 1989 was the Adrenal Stress Index (ASI). It truly constitutes a chronobiologic investigation. It includes four cortisol evaluations throughout the day: fasting (on awakening), noon, late afternoon, and bedtime, to discover any disturbances to the circadian cortisol rhythm. Chronobiologic salivary testing has been used clinically most prominently by the US government. Hormone test panels that measure cortisol and other hormones (from Diagnos-Techs) have been relied upon clinically to assess objectively the circadian adaptation of aircrew to transmeridian flight, to create physiologic profiles of soldiers experiencing military survival training, and to evaluate circadian shifts in astronauts before flight (including the proposed Mars Project astronauts).21-24
Cycling Woman's Hormone Assessment
A prime example of chronobiologic evaluation is a cycling female hormone assessment. Because of the wide variations of the two principal cycling hormones, estradiol and progesterone, throughout the follicular and luteal phases of a woman's monthly cycle, no single day's hormonal sample can represent reproductive hormone status. Chronobiologic study entails collection of saliva every 2 to 4 days, depending on the length of the woman's cycle. An accurate evaluation of the vagaries of a woman's hormones throughout her cycle can be presented (See Figure 1). Expanding the study adds seven periovulatory measurements of the pituitary hormones, follicular stimulating hormone (FSH), and luteinizing hormone (LH). These assessments are valuable particularly for women experiencing fertility problems, premenopausal symptoms, and menopausal distress.
Figure 1
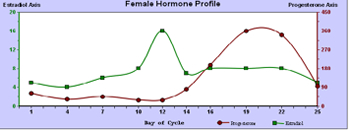
Bone Health Assessment
Salivary hormone assessment can be combined with other measurements to provide a more complete picture of a clinical condition, as well as a more scientific basis for knowledgeable therapy. Although the pathogenic events involved in the development of osteopenia and its progress to osteoporosis are relatively irreversible, they can be slowed down and ameliorated by early detection and intervention. Traditionally, diagnosis has relied on bone densitometry (mineralized bone mass assay) using radioactive or radiographic techniques performed at a referral facility. Noninvasive methods to estimate bone mineral density (BMD) include a number of specific urinary and serum assays of bone resorption markers for the diagnosis and follow-up of osteoporotic patients. Assays of Pyrilinks-D (DPD), pyridinoline, and N-terminal cross-linked peptides (NTx) in serum and urine have been advanced as sensitive and specific markers for bone resorption. Pyrilinks-D is specific for bone resorption, and does not measure collagen degradation; it has a 96% to 98% range of accuracy.25-29 Pyrilinks-D assay has demonstrated precision and accuracy equal or superior to NTx and linear C telopeptides of type I collagen (CTx).25,29 It is a better predictor of bone loss than invasive serum osteocalcin measurements.27 As well, Pyrilinks-D measurements correlate well with treatment.26,29,30
Although a noninvasive Pyrilinks- D urinary assay may be obtained separately, a basic metabolic study should be added by including an assessment of hormones that underlie bone metabolism. These include estradiol, progesterone, testosterone, cortisol, DHEA-DHEAS, and FSH. The information provided constitutes a valuable database upon which to construct an appropriate treatment plan for patients with osteopenia and osteoporosis. Appropriate correction of hormonal imbalance, coupled with calcium and vitamin supplementation, and use of various pharmacological interventions, can provide the underpinning of successful therapy.
Andropause Assessment
It is becoming more evident that investigation of hypogonadism in men, sometimes termed andropause, cannot be limited to a single serum testosterone assessment. Not only is such a measurement unreliable and fraught with error, as previously noted, but it does not account for the daily and weekly circadian rhythms of testosterone (common to most sex hormones). As well, overall problems with deficiencies of antecedent and predecessor hormone supply, capability of the underlying testosterone hormone metabolic co-reactions, the amount present of the potent male hormone dihydrotestosterone, and the dynamics of the controlling pituitary hormones FSH and LH should be taken into account prior to any possible recommendations for testosterone therapy. Moreover, aromatization of testosterone to estradiol and conversion of androstenedione to estriol and estradiol (common to older men) are not accounted for without measurements for all these hormones. These values should be known prior to pronouncing the testes unresponsive, and prescribing testosterone therapy. A representative and thorough investigation of the androgen pathway can provide a sound basis for therapy recommendations (Figure 2).
Figure 2
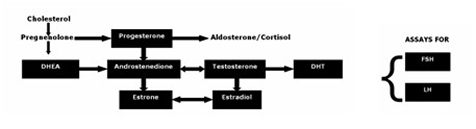
Antibody Assessment
In addition to hormone assays, saliva has proven valuable for antibody assessment, especially of the immunoglobulins. Many clinicians rely upon serum IgA, IgE, IgG, and IgM antibody evaluations to help diagnose the presence of food sensitivities, parasites, and associated problems. Most of these antibody responses originate in the blood compartment, and are activated only latterly upon antigen exposure. Saliva, which reflects activities in the tissue fluid compartment, can respond sooner to an antigen exposure and has no "memory; that is, antibodies wane within 1 to 2 months after an antigen exposure is removed (particularly IgA). This property allows sequential salivary antibody assessments to be used not only to gauge the presence of an antigen, but also to assess the effectiveness of therapy and how well a patient adheres to specific dietary restrictions.
IgA is a dimer produced primarily by mucosal cells that predominate in the GI tract and, to a lesser degree, the respiratory and genitourinary tracts. In the gut, IgA originates mainly from local tissue immunocytes, especially in the lamina propria of the intestines. It is responsible for immune exclusion, the first line of defense wherein intestinal IgA reduces the uptake of food antigens, bacterial toxins, and several other macromolecules. Within intestinal secretions in the mucosa, locally produced IgA can complex the antigens, reducing their penetration and reactivity. IgA antibodies can prepare monocytes and lymphocytes for cytotoxicity against bacteria and can render them "microphiles, increasing retention within and washout from the gut.
This reactive and protective effect of IgA antibodies is especially active and pertinent in the gut, the major source of dimeric IgA. It follows that measurement of IgA antigens and antibodies provides a meaningful assessment of food, bacterial, and parasitic problems of the gut. Because of these specific diagnostic properties, IgA analysis holds promise as the best way to diagnose many of these common problems. The level of IgA in stool has an 86% to 92% correlation with intestinal production of IgA (as far proximal as the duodenum). Derangements in IgA production may herald gut malabsorption, food sensitivity, and candidiasis, or parasitic infections. Additionally, assessment of selected salivary IgA antibodies can explore food sensitivities to milk, soy, egg, and gluten, as well as ameba, Helicobacter, Toxoplasma, tapeworm, roundworm, and tissue worms.
Conclusion
The examples presented in this article illustrate how comprehensive and applicable saliva assays are, alone and in combination with associated testing, and that they provide a most complete assessment of a clinical problem as currently possible. With its proven accuracy, ease and privacy of collection, avoidance of venipuncture, low cost, and ability for chronobiologic assessment, saliva testing is a distinct improvement over serum testing. Given the increasing awareness of these benefits, its clinical use is increasing rapidly.

 John J. White, MD, CM, received his degree from McGill University in Montreal, spent two years active duty with the US Coast Guard (US Public Health Service), and thereafter qualified in general surgery and cardiovascular and thoracic surgery at the McGill University Teaching Hospitals. He further qualified in pediatric surgery at Johns Hopkins, spent 10 years on staff there, and finished his academic career as a professor of pediatric surgery at various institutions. Dr. White is the author of over 175 peer-reviewed scientific publications as primary or coauthor. After retiring in Seattle near his children and grandchildren, Dr. White is pleased to have a second career at Diagnos-Techs. Recently, he was appointed medical director of the Medical Support Group for Diagnos-Techs. John J. White, MD, CM, received his degree from McGill University in Montreal, spent two years active duty with the US Coast Guard (US Public Health Service), and thereafter qualified in general surgery and cardiovascular and thoracic surgery at the McGill University Teaching Hospitals. He further qualified in pediatric surgery at Johns Hopkins, spent 10 years on staff there, and finished his academic career as a professor of pediatric surgery at various institutions. Dr. White is the author of over 175 peer-reviewed scientific publications as primary or coauthor. After retiring in Seattle near his children and grandchildren, Dr. White is pleased to have a second career at Diagnos-Techs. Recently, he was appointed medical director of the Medical Support Group for Diagnos-Techs.

Notes
1. Denny P, Hagen FK, Hardt M, et al. The proteomes of human parotid and submandibular/sublingual gland saliva collected as the ductal secretions. J Proteome Res. 2008;10:1012.
2. Wong DT. Salivary Diagnostics Powered by Nanotechnologies, Proteomics, and Genomics J Am Dent Assoc. 2006;137:313–321.
3. Rosner W, Auchas RJ, Azziz R. utility, limitations, and pitfalls in measuring testosterone: an Endocrine Society position statement. J Clin Endocrinol Metab. 2008; 92:405–413.
4. Rosner W, Vesper H. Toward excellence in testosterone testing: a consensus statement. J Clin Endocrinol Metab. 2010) 95:4542–4548.
5. Wartofsky L, Handelsman DJ. Standardization of hormonal assays for the 21st century. J Clin Endocrinol Metab. 2010) 95:5141–5143.
6. Gardner D, Shoback D. Greenspan's Basic & Clinical Endocrinology. 8th ed. McGraw-Hill; 2007.
7. Peters JR, Walker RF, Riad-Fahmy D, et al. Salivary cortisol assays for assessing pituitary-adrenal reserve. Clin Endocrinol. 1982;17:583–592.
8. Vining RF et al. Salivary cortisol: a better measure of adrenal cortical function than serum cortisol. Ann Clin Biochem. 1983;(Pt6):329–335.
9. Vining RF, McGinley RA, Symons RG. Hormones in saliva: mode of entry and consequent implications for clinical interpretation. Clin Chem. 1983;29:1752–1756.
10. Read GF, Walker RF, Wilson DW, et al. Steroid analysis in saliva for the assessment of endocrine function. Ann N Y Acad Sci. 1990) 595:260–274.
11. Malamud D. Saliva as a diagnostic fluid. Brit Med J. 1992;305:207–208.
12. Ellison PT. Salivary steroids and natural variation in human ovarian function. Ann N Y Acad Sci. 1994;709:287–298.
13. Collins JJ. Salivary hormone testing: science, benefits, limitations & clinical applications. Anti-Aging Medical News. Winter 2000.
14. Lewis JG. Steroid analysis in saliva: an overview. Clin Biochem Rev. 2006) 27:139–146.
15. Gröschl M. Current status of salivary hormone analysis. Clin Chem. 2008;54:11 1759–1769.
16. Raff H. Utility of salivary cortisol measurements in Cushing's syndrome and adrenal insufficiency. J Clin Endocrinol Metab. 2009;94:3647–3655.
17. Arafah BM, Nishiyama FJ, Tlaygeh H, et al. Measurement of salivary cortisol concentration in the assessment of adrenal function in critically ill subjects: a surrogate marker of the circulating free cortisol. J Clin Endocrinol Metab. 2007;92:2965–2971.
18. Gozansky WS, Lynn JS, Laudenslager ML, et al. Salivary cortisol determined by enzyme immunoassay is preferable to serum total cortisol for the assessment of dynamic hypothalamic—pituitary—adrenal axis activity. Clin Endocrinol (Oxf). 2005;63:336–341.
19. Gavrilova N, Lindau ST. Salivary sex hormone measurement in a national, population-based study of older adults. J Gerontol B Psychol Sci Soc Sci. 2009;64 Suppl 1:i94–105.
20. Nieman LK, Biller BM, Findling JW, et al. The diagnosis of Cushing's syndrome: an Endocrine Society clinical practice. J Clin Endocrinol Metab. 2008)93:1526–1540.
21. French J, Bisson RU, Neville KJ, et al. Circadian adaptation of aircrew to transmeridian flight. Aviat Space Environ Med. 1994)65(5Suppl):A1–6.
22. Morgan CA III, Wang S, Mason J, et al. Hormone profiles in humans experiencing military survival training. Bio Psychiatry. 2000)47:891–901.
23. Whitson PA, Putcha L, Chen YM, et al. Melatonin and cortisol assessment of circadian shifts in astronauts before flight. J Pineal Research. 1995)18:141–147.
24. Assessing Group Dynamics in a Mars Simulation. 2006. Project Site: NASA Haughton-Mars Project (HMP) on Devon Island, Canada.
25. Ross PD, Knowlton W. Rapid bone loss is associated with increased levels of biochemical markers. J Bone Miner Res.. 1998)13:297–302.
26. Fassbender WJ, Gödde M, Brandenburg VM, et al. Urinary bone resorption markers (deoxypyridinoline and c-terminal telopeptides of type I collagen) in healthy persons, postmenopausal osteoporosis and patients with type I diabetes. Adv Med Sci.. 2009)54:1–6.
27. Lenora J, Ivaska KK, Obrant KJ, et al. Prediction of bone loss using biochemical markers of bone turnover. Osteoporos Int. 2007;18:1297–1305.
28. Al-Awadi A, Olusi SO, Al-Zaid N, et al. Serum B2-microglubulin concentration correlates with urinary concentrations of type I collagen cross-linked N-telopeptides and deoxypyridinoline in rheumatoid arthritis. Ann Saudi Med. 1998)18:113–116.
29. Yu SL, Ho LM, Lim BC, et al. Urinary deoxypyridinoline is a useful biochemical bone marker for the management of postmenopausal osteoporosis. Ann Acad Med Singapore. 1998;27:527–529.
30. Palomba S, Orio F Jr, Colao A, et al. Effects of estrogen replacement plus low-dose alendronate treatment on bone density in surgically postmenopausal women with osteoporosis. J Clin Endocrinol Metab. 2002;87:1502–1508.


|
|



![]()
![]()
![]()



 John J. White, MD, CM, received his degree from McGill University in Montreal, spent two years active duty with the US Coast Guard (US Public Health Service), and thereafter qualified in general surgery and cardiovascular and thoracic surgery at the McGill University Teaching Hospitals. He further qualified in pediatric surgery at Johns Hopkins, spent 10 years on staff there, and finished his academic career as a professor of pediatric surgery at various institutions. Dr. White is the author of over 175 peer-reviewed scientific publications as primary or coauthor. After retiring in Seattle near his children and grandchildren, Dr. White is pleased to have a second career at Diagnos-Techs. Recently, he was appointed medical director of the Medical Support Group for Diagnos-Techs.
John J. White, MD, CM, received his degree from McGill University in Montreal, spent two years active duty with the US Coast Guard (US Public Health Service), and thereafter qualified in general surgery and cardiovascular and thoracic surgery at the McGill University Teaching Hospitals. He further qualified in pediatric surgery at Johns Hopkins, spent 10 years on staff there, and finished his academic career as a professor of pediatric surgery at various institutions. Dr. White is the author of over 175 peer-reviewed scientific publications as primary or coauthor. After retiring in Seattle near his children and grandchildren, Dr. White is pleased to have a second career at Diagnos-Techs. Recently, he was appointed medical director of the Medical Support Group for Diagnos-Techs.