|
Lyme disease is the most prevalent tick-borne disease in the US. The Centers for Disease Control and Prevention (CDC) reported nearly 32,500 new cases in 2011, though it is estimated that the actual number could be up to 10-fold higher, making Lyme disease an epidemic larger than AIDS, West Nile virus, and Avian flu combined.1–5
Unfortunately, only a fraction of these cases are being treated due to equivocal clinical manifestations, inaccurate tests, and underreporting.2 Patients not receiving adequate treatment may develop chronic infection or late-stage Lyme diseases such as chronic Lyme arthritis or chronic Lyme neuroborreliosis, which can be devastating in some cases.6
Lyme disease is caused by Borrelia burgdorferi, a bacterium of the spirochete class. Lyme disease is a zoonotic, vector-borne disease transmitted by the Ixodes (blacklegged) tick. Symptoms may include observed tick bite, a "bulls-eye" rash, flu-like symptoms, joint pain, neurological symptoms, heart palpitations, and severe fatigue.
The current CDC-recommended evaluation for diagnosis is a two-tier test, including ELISA and western blot analyses. These tests are serological assays that detect antibodies to B. burgdorferi. The low sensitivity of the two-tier tests (about 30% in early Lyme disease and 50% in late Lyme disease) and the significant seronegativity of Lyme patients (as many as 30% to 50% of cases) suggests that more sensitive T cell-based laboratory tests should also be developed.7 A thorough evaluation for Lyme requires testing for a humoral and a cellular immune response. This is done by measuring both antibodies (humoral/WB) and T cell activity (cell-mediated/ELISPOT).
Enzyme-linked immunosorbent spot (ELISPOT) is an effective method for assessment of the magnitude and the quality of T cell immunity by measuring stimulated antigen-specific T cells.8,9 This method and over 100 tests that measure nervous-, endocrine-, and immune-system markers are performed by Pharmasan Labs in a state-of-the-art, CLIA-certified specialty reference laboratory with 12 PhDs on staff. Combined with NeuroScience’s custom health solutions, Pharmasan Labs offers natural practitioners turnkey solutions to effectively address the root causes of patient' conditions.
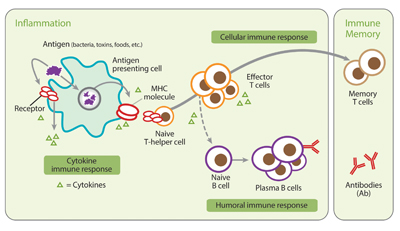
Figure 1:Inflammatory immune response: Individuals who have been infected with B. burgdorferi harbor B. burgdorferi-specific immune cells (T cells) in their bloodstream. Typically, these T cells can be detected before an antibody response.
Clinical Relevance
The ELISPOT method utilized in iSpot Lyme is a highly sensitive technique for detecting immune cells that secrete signature proteins (such as a given cytokine). It is the only available technology that accurately detects, measures, and performs functional analysis of low-frequency immune cells. The sensitivity of ELISPOT is much higher than that of ELISA and the flow cytometry-based intracellular cytokine staining method.10 iSpot Lyme has a sensitivity of 84% and specificity of 94%.
The iSpot Lyme test detects a cellular immune response against Lyme antigens, which appears earlier in the disease process than the antibody response detected by the traditional western blot test.11 More importantly, iSpot Lyme can detect antigen-specific T cell responses in seronegative patients.12 Therefore, the Lyme ELISPOT test can be used to provide information regarding the current immune status of a Lyme disease patient.
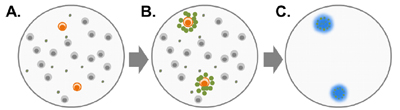
Figure 2: When peripheral blood mononuclear cells (PBMCs) from a B. burgdorferi-infected patient are exposed to B. burgdorferi protein antigens (A), B. burgdorferi-specific T cells are activated and secrete small proteins called cytokines (B). T cells that are not specific for B. burgdorferi do not become activated. iSpot Lyme measures the cytokine IFN-g secreted by the patient's T cells. Cytokine proteins (IFN-g) are captured near the cells that secreted them, and are then detected using a color reagent (C).
iSpot Lyme Test
The iSpot Lyme test detects B. burgdorferi-specific T cell responses in patients who have been exposed to B. burgdorferi spirochetes. Individuals who have been infected with B. burgdorferi harbor B. burgdorferi-specific immune cells (T cells) in their bloodstream. Typically, these T cells can be detected before an antibody response.
The result is produced by measuring IFN-g secreted by T cells in response to stimulation by the B. burgdorferi antigens DbpA, OspC, p100, and VisE-1. This test measures frequency of antigen-specific T cells by measuring T cells that are specific for Lyme antigens. This is indicative of exposure to Lyme. A single result is provided on the report.
Methodology
The immune response to infection with B. burgdorferi includes both B cell and T cell activation.13,14 T cells are sensitized to B. burgdorferi antigens and the activated effector T cells produce the cytokine interferon gamma (IFN-g) when stimulated by these antigens. The iSpot Lyme test counts B. burgdorferi-sensitized T cells by capturing interferon-gamma (IFN-g) secreted by these T cells. More specifically, when IFN-g is released a "spot" of insoluble precipitate is formed at the site of the reaction. Evaluating the number of spot forming units (SFUs) provides a measurement of B. burgdorferi-sensitive effector/memory T cells in the peripheral blood. The SFU count correlates to a patient’s T cell reaction to B. burgdorferi.
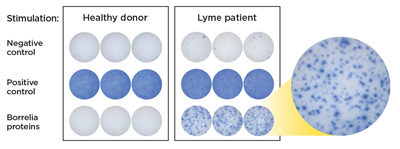
Figure 3: The resulting spot forming units (SFUs) represent individual B. burgdorferi -reactive T cells that are counted to determine a positive or negative test result.
Conclusion
Lyme disease is an increasingly common condition that has varying stages of severity. A comprehensive approach to diagnosis will lead to better treatments and outcomes. Pharmasan Lab' iSpot Lyme test is a highly sensitive test to identify B. burgdorferi infection, aiding health-care professionals to better diagnoses of their patients.
Notes
1. Reported cases of Lyme disease by year, United States, 1995-2013 [Web page]. CDC. http://www.cdc.gov/lyme/stats/chartstables/casesbyyear.html.
2. Yound JD. Underreporting of Lyme disease. N Engl J Med. 1998. 338(22):1629–1629.
3. Statistics overview [Web page]. CDC. http://www.cdc.gov/hiv/topics/surveillance/basic.htm.
4. West Nile virus [Web page]. CDC. http://www.cdc.gov/ncidod/dvbid/westnile/surv&controlCaseCount12_detailed.htm.
5. Cumulative number of confirmed human cases for avian influenza A(H5N1) reported to WHO, 2003-2012 [online document]. WHO. http://www.who.int/influenza/human_animal_interface/EN_GIP_20121217
CumulativeNumberH5N1cases.pdf
6. Maloney EL. The need for clinical judgment in the diagnosis and treatment of Lyme disease. J Am Physicians Surg. 2009. 14(3):82–89.
7. Lehmann PV, Zhang W. Unique strengths of ELISPOT for T cell diagnostics. In: Kalyuzhny A, ed. Handbook of ELISPOT: Methods and Protocols. Vol. 792. Methods in Molecular Biology. 2nd ed. New York: Spriner Science+Business Media, LLC; 2012: 3–23.
8. T-Spot. TB 96 [package insert]. Oxfordshire, UK: Oxford Immunotec Limited; 2009.
9. Tary-Lehmann M, Hamm CD, Lehmann PV. Validating reference samples for comparison in a regulated ELISPOT assay. In: Orabhakar U, Kelley M, eds. Validation of Cell-Based Assays in the GLP Setting: A Practical Guide. 1st ed. West Sussex, UK: John Wiley & Sons Ltd; 2008:127–146.
10. Forsberg P, Ernerudh J, Ekerfelt C, et al. The outer surface of Lyme disease borrelia spirochetes stimulate T cells to secrete interferon-gamma (IFNg): diagnostic and pathogenic implications. Clin Exp Immunol. 1995;101:453–460.
11. Ekerfelt C, Forsberg P, Svenvik M, et al. Asymptomatic Borrelia-seropositive individuals display the same incidence of Borrelia-specific interferon-gamma (IFN-g)-secreting cells in blood as patients with clinical Borrelia infection. Clin Exp Immunol. 1999;115:498–502.
12. Dattwyler RJ, Volkman DJ, Luft BJ, et al. Seronegative Lyme disease – dissociation of specific T- and B-lymphocyte responses to Borrelia burgdorferi. N Engl J Med. 1988;319(22):1441–1446.
13. Dressler F, Yoshinari NH, Steere AC. The T-cell proliferative assay in the diagnosis of Lyme disease. Ann Intern Med. 1991;115:533–539.
14. Martinuzzi E, Scotto M, Enee E, et al. Serum-free culture medium and IL-7 costimulation increase the sensitivity of ELISpot detection. J Immunol Methods. 2008;333:61–70.
 Bradley Bush, ND Bradley Bush, ND
Dr. Bush’s background provides insightful understanding of the natural health marketplace. His experience includes licensed naturopathic doctor, marketing director, salesperson, and health-care consultant for the nutritional supplement and laboratory testing industries. Currently in private practice in Stillwater, Minnesota, Dr. Bush formerly served as director of clinician affairs for NeuroScience Inc. Committed to advancing education in the natural health sciences, Dr. Bush is a sought-after author and presenter on naturopathic and integrative medicine topics.
|
![]()
![]()
![]()








 Bradley Bush, ND
Bradley Bush, ND