Page 1, 2
Managing Galectin 3 Levels with Modified Citrus Pectin
Galectin-3 inhibition
The research has made it clear that increased levels of Gal-3 are problematic in inflammation and in several pathologies, contributing to a shorter lifespan. It is clear that inhibiting and reducing the activity and levels of Gal-3 improves outcomes in many acute and chronic conditions.
Although there are currently several Gal-3-inhibiting pharmaceuticals being studied, it will be five years or more, apparently, before any of these medications reach the market. In short, there are currently no pharmaceuticals approved for the management of Gal-3 levels.
 The only Gal-3 inhibitor on the market at this time is modified citrus pectin (MCP), a nutritional supplement that appears to work quite well. We owe thanks to Dr. Isaac Eliaz for the formulation, testing, and commercial introduction of modified citrus pectin (MCP) two decades ago and for the clinical training he provides on the importance of Gal-3 testing. Many of the pathological processes promoted by Gal-3 can be effectively managed with modified citrus pectin. The form of pectin used in MCP is the white pith in citrus peels, modified with enzymatic processes to lower molecular weight and degree of esterification. Modification of the pectin allows the MCP to be absorbed out of the gut and increases bioavailability to bind with Gal-3 and block its harmful effects. The only Gal-3 inhibitor on the market at this time is modified citrus pectin (MCP), a nutritional supplement that appears to work quite well. We owe thanks to Dr. Isaac Eliaz for the formulation, testing, and commercial introduction of modified citrus pectin (MCP) two decades ago and for the clinical training he provides on the importance of Gal-3 testing. Many of the pathological processes promoted by Gal-3 can be effectively managed with modified citrus pectin. The form of pectin used in MCP is the white pith in citrus peels, modified with enzymatic processes to lower molecular weight and degree of esterification. Modification of the pectin allows the MCP to be absorbed out of the gut and increases bioavailability to bind with Gal-3 and block its harmful effects.
Cardiovascular Health
The published research on MCP has shown that by managing galectin-3, this supplement modulates immune activity and reduces chronic inflammation:
- Improving cardiovascular function,
- Reversing fibrosis in heart disease and in liver and kidney disorders,
- Preventing progression of atrial fibrillation,
- Removing toxic minerals safely without removing essential minerals.
Cancer Management
MCP acts to fill the binding sites on the Gal-3 molecule, thus inhibiting the ability of this lectin to bind to tissue and toxins, rendering Gal-3 less able to block normal function or accelerate inflammation. (These effects are not generally observed with the food pectin, which typically occurs in fruits. Unmodified pectin is not absorbed in the GI tract and, therefore, has no observable metabolic effects.)
Modified Citrus Pectin for Cancer Support
MCP provides the following:
- Immune support;
- Blocking of Gal-3;
- Slowed cancer progression and metastasis;
- Synergistic effects with chemotherapy, botanicals, and prostate support products;
- Synergistic effects with radiation therapy;
- Protection against post-radiation damage;
- Improved quality of life at all stages.
MCP reduces the following:
- Primary tumors,
- Angiogenesis,
- Chronic inflammation,
- Metastatic processes,
- Heavy metals and toxins.
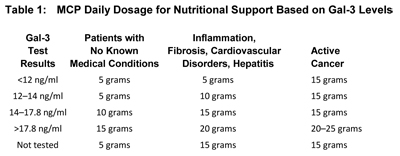
Table 1, based on the work of Isaac Eliaz, MD, offers specific dosage information, with the following caveats:
- In monitoring a patient who is generating high levels of inflammation, the Gal-3 test results may change slowly, but the Gal-3 inhibitor (modified citrus pectin) will block the effects of inflammation, and clinical improvement will still occur.
- For biopsy or surgery, the patient should take the full dosage up to surgery and immediately following surgery.
- In cases of active cancer, chemotherapy, radiation, or hormonal therapy, the dosage should be 5 grams, three times per day, up to 25 grams daily, based on Gal-3 levels.
- In post-cancer treatment, maintain MCP supplementation for the first five years of remission (the period of highest risk), at a full dosage of 5 grams three times a day. After 5 years, continue the dosage based on Gal-3 levels and on the patient's condition.
- In cases of advanced disease, such as metastatic cancer, Gal-3 levels may not change significantly, but the modified citrus pectin can slow the progress of disease and support quality of life.
Is MCP for Everyone?
Who should take MCP, in addition to those with heart disease, cancer, or other pathologies discussed here? The research literature indicates that Gal-3 levels begin increasing in our 20s and 30s, indicating a reduction in the ability to lower inflammation and fibrosis and support regeneration. This is apparently an aspect of the aging process. Consequently, based on this finding, Dr. Eliaz suggests that everyone over the age of 30 take MCP. Modified citrus pectin works well with other supportive remedies, both pharmaceutical and natural, and is a good fit for general health and anti-aging programs.
Antiaging
A Personal Note From the Author: Early in my career in the 1970s, I was involved in a study of what it would take to significantly increase human lifespan. One conclusion was that effective clinical management of longevity would require greatly improved analytical tools, evaluating very deep specifics of metabolism supported by therapies that manage the biochemical dynamics of the aging body using interventions that do not create additional problems. Functional medicine as a whole reflects this drive for better analytics to monitor metabolic function, not just to confirm the presence of disease. The Gal-3 test is this type of analytical tool. It evaluates risk, stage of pathology, and functional processes and is readily managed with available, well-tolerated nutritional supplements.
Conclusion
Galectin-3 induction is an aspect of many essential repair functions in infection and injury. There are numerous papers indicating the role of Gal-3 in healthy, early immune response, demonstrated both in vitro and in animal studies. It appears that if the injury is chronically repeated or if infections are chronic, Gal-3 is overexpressed and becomes an inflammatory and fibrotic promoter that is problematic. While we may not always know precisely why this transition occurs, it is important to monitor these levels and identify the need for Gal-3 inhibition with readily available nutritional solutions.
The Range of Galectin-3 Dynamics
Although elevated plasma levels of Gal-3 can be degenerative, intracellular levels have been found to be protective. Intracellular Gal-3, produced locally within the kidney tissue, for example, is strongly expressed within the ureteric bud and associated tissue, contributing to normal nephrogenesis. Thus intracellular Gal-3 can have a regenerative affect.
However, plasma levels of Gal-3, when high, contribute to rapid loss of kidney function. When over-expressed, Gal-3 contributes to end-stage organ damage in the kidneys, resulting in fibrosis and sclerosis. (Aldosterone causes Gal-3 expression and release.) In most cases, it is the fibrosis that leads to kidney disease. Data from the LURIC (Ludwigshaven Risk and Cardiovascular Health) study and 4D (Deutsche Diabetes Dialysis study) show clear association between higher levels of Gal-3 and the stage of kidney disease. Levels above 23.1 are associated with stage 3a to stage 5, with progressively lower glomerular filtration rate (eGFR) levels and impaired kidney function.
The number of Americans with diagnosed kidney disease is approximately 4.5 million, but the estimated number of those unaware of impaired kidney function is estimated at 10 to 20 million. At least 7.4 million people have less than half the normal kidney function of a young adult. Given the role of kidney function in progression toward more severe diabetic and cardiovascular disease, Gal-3 testing and management could become crucial factors in health maintenance. An important paper published in the American Journal of Nephrology (2016) concluded: "…inhibition of galectin-3 may be a promising therapeutic strategy to prevent end-stage kidney disease."
Page 1, 2
References
Chen SC, Kuo PL. The role of galectin-3 in the kidneys. Int J Mol Sci. April 14, 2016;17(4):565.
de Boer RA, van Veldhuisen DJ, Gansevoort RT, et al. The fibrosis marker galectin-3 and outcome in the general population. J Intern Med. July 2012;272(1):55-64.
Desmedt V, Desmedt S, Delanghe JR, Speeckaert R, Speeckaert MM. Galectin-3 in renal pathology: More than just an innocent bystander.Am J Nephrol. 2016;43(5):305-17.
Eliaz I. Galectin-3: The Aging Biomarker (webinar). Available at www.betterhealthpublishing.com. Accessed 10-01-16.
Gleissner CA, Erbel C, Linden F, et al. Galectin-3 binding protein plasma levels are associated with long-term mortality in coronary artery disease independent of plaque morphology. Atherosclerosis. August 2016;251:94-100.
Hättasch R, Spethmann S, de Boer RA, et al. Galectin-3 increase in endurance athletes. Eur J Prev Cardiol. October 2014;21(10):1192-9.
Henderson NC, Sethi T. The regulation of inflammation by galectin-3. Immunol Rev. July 2009;230(1):160-71.
Martínez-Martínez E, Calvier L, Rossignol P, et al. Galectin-3 inhibition prevents adipose tissue remodelling in obesity. Int J Obes (Lond). June 2016;40(6):1034-8.
van der Velde AR, Gullestad L, Ueland T, et al. Prognostic value of changes in galectin-3 levels over time in patients with heart failure: data from CORONA and COACH. Circ Heart Fail. March 2013;6(2):219-26.
van der Velde AR, Meijers WC, van den Heuvel ER, et al. Determinants of temporal changes in galectin-3 level in the general population: Data of PREVEND. Int J Cardiol. November 1, 2016;222:385-90.
 Author: Jerry Stine Author: Jerry Stine
Jerry Stine is a nutritional consultant and the director of the Lifespan Institute for Functional and Anti-Aging Nutrition, which he founded in 1987 to develop advanced life-extension and performance-enhancement programs. For the past 20 years, he has been an independent nutritional counselor with an active private practice and has served as consultant for several respected vitamin manufacturers. For clinical consultations or additional information, he can be reached by phone at 707-421-2143 or at lifespan2@comcast.net.
 Editorial: Nancy Faass Editorial: Nancy Faass
Nancy Faass, MSW, MPH, is a writer and editor in San Francisco who has worked on more than 50 books for publishers that include Elsevier, Harper, McGraw Hill, and others. Director of the Health Writers' Group for the past 20 years, she works collaboratively with clients to develop articles, Web content, white papers, blogs, manuals, and books and can be reached by emailing info@HealthWritersGroup.com or calling 415-922-6234.
Resources
Sources of Galectin-3 Testing:
LabCorp.com and Mayo Medical Laboratories
Sources of Modified Citrus Pectin:
Clinical Synergy at clinicalsynergyformulas.com or 707-303-1999
Econugenics at econugenics.com or 800-308-5518
Allergy Research Group at allergyresearchgroup.com or 800-545-9960
Lifespan Products LLC at lifespanproducts.com or 707-421-2143
Links to Dr. Eliaz Webinars:
www.betterhealthpublishing.com/webinars-view/
Password: DrIsaacEliaz |
![]()
![]()
![]()
![]()







 Author: Jerry Stine
Author: Jerry Stine Editorial: Nancy Faass
Editorial: Nancy Faass