|
www.cancerdecisions.com
How Goes the War?
December 2011 will mark the 40th anniversary of the official US government war on cancer. On various editorial pages, you can expect to hear fulsome praise of the wonderful progress that has been made over the past four decades. We will repeatedly be reassured that our $100+ billion investment in cancer research was well worth it. It has enriched our understanding of the malignant disease process, etc. Cures are emerging and if we just hang in there for a few more years, the much sought-after cancer cure will be at hand. The public – promised a cure in time for the American bicentennial, July 4, 1976 – will be left with the impression that these scores of billions of dollars have brought us, if not an actual cure, at least a giant step closer to that ardently desired goal.
My own view is more skeptical. Of course, there are some bright areas. You can hardly set some of the world's brightest scientists to work on a problem, give them billions of dollars in funding, and come up completely empty-handed. But, in my heart, my conclusion is little different from that expressed by Linus Pauling, PhD, in a jacket quote for my 1980 book, The Cancer Industry. He wrote, "Everyone should know that most cancer research is largely a fraud and that the major cancer research organizations are derelict in their duties to the people who support them."
Over the course of this year, leading up to the actual 40th anniversary, I would like to look at some of the results of the war on cancer. In this article I will focus on lung cancer. I do this for a number of reasons. First, cancer of the lung and bronchus is a tragically common in our society. In 2010 there were 220,520 new cases in the US. It thus edges out prostate cancer (with 217,730 new cases annually) as well as breast cancer (209,060 new cases) as the most common form of life-threatening malignancy. It is also by far the most deadly, killing 157,300 Americans per year (compared with 40,230 for breast and 32,050 for prostate. All figures are from the American Cancer Society's "Cancer Facts & Figures 2010").
Another reason for choosing lung cancer is a technical one. There is currently no standard screening test for this disease. This is unfortunate for potential victims (such as tobacco smokers, who account for about 80% of the patient base). But it does convey one benefit for students of cancer statistics. The introduction of new detection technologies (such as mammography for breast cancer and the prostate specific antigen, or PSA, test for prostate cancer) tends to recruit a large group of new patients into the statistical columns. The results in a phenomenon called lead-time bias.
Lead-time is the length of time between the point at which a cancer is detected by modern tests and the point at which it would formerly have been clinically detected. Lead-time bias "extend[s] the statistical length of a patient's survival without necessarily prolonging the duration of life," according to the late Alvan R. Feinstein, PhD, of Yale University. The earlier the cancer is found, the longer the patient thus "survives." In actuality, you understand, no actual time has been added to the patient's lifespan. But on paper, there has been great improvement.
Lead-time bias accounts for much of the illusory "improvement" seen over the last few decades in treating breast, prostate, and a few other kinds of cancer wherein the patient population has increased greatly. Patients are actually dying with the same regularity. But the statistics look a whole lot better. So, as we survey the possible improvement in cancer treatment over the past 40 years, it is more realistic to look at a type of cancer (i.e., lung/bronchus) in which lead-time bias is less likely to distort the true statistical picture.
My first question concerns the 5-year relative survival odds in patients with lung cancer.
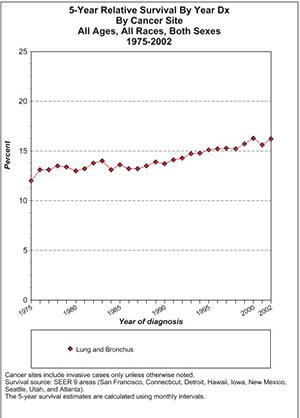
This is a chart generated by the government's SEER website (Surveillance Epidemiology and End Results; SEER.cancer.gov). It analyzes survival data in nine US areas (San Francisco, Connecticut, Detroit, Hawaii, Iowa, New Mexico, Seattle, Utah, and Atlanta) from which we can presumably extrapolate to what has happened in the US as a whole.
In Table 1, one can see the 5-year survival among patients of all ages, all races, and both sexes. The starting point is a 13 or so percentage-point 5-year survival. By 2002 the 5-year relative survival is around 16%, or an improvement of a few percentage points. I would ascribe any improvement to the general improvement in medical and ancillary care, not to cancer treatment per se.
We can also look at a group that is particularly prone to this type of cancer, men aged 50-plus who were diagnosed with lung cancer at the beginning of the war on cancer and in more modern times. To answer this we can look at Table 2.
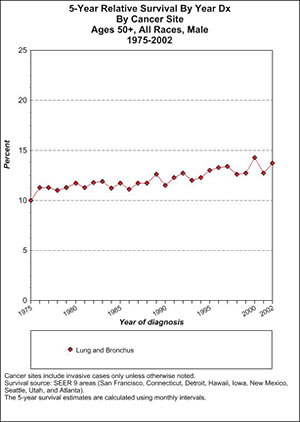
This shows that 5-year survival was attained by 10% of older male lung cancer patients in 1975. In the last year for which SEER provides data, 2002, it is about 14%. Thus, there was around a 4% improvement in 5-year survival over the first quarter century of the war on cancer.
This is pretty extraordinary, when you think of it. After all, medicine as a whole has progressed mightily since 1975. In 1975, there were no routine CT scans, or many of the other life-saving measures that we take for granted today. Yet, despite all that, the 5-year survival rate in the most common patient type has moved only a few percentage points. (I am assuming that present-day trends are similar to what was seen until 2002.)
If we look at lung cancer survival in general, the picture is equally grim (Table 3).
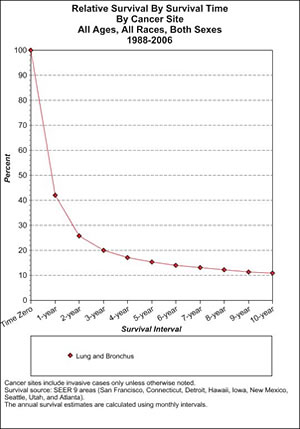
Here we see how precipitously survival falls off for patients with cancer of the lung or bronchus. (These are the latest figures available, for 1988–2006). In the first 2 or 3 years they simply fall off a cliff. But as time progresses, patients continue to die of the disease, falling to a mere 10% surviving at 10 years. Again, this is despite all the many supposed improvements in cancer care brought to us by the war on cancer.
Drugs Approved for Lung Cancer
The past 40 years have certainly seen the introduction of numerous anticancer agents for lung cancer. In the past decade and a half, the Food and Drug Administration (FDA) has approved the following for first-line treatment of non-small cell lung cancer: navelbine, paclitaxel, gemcitabine, and docetaxel. In addition, docetaxel was also approved for second-line treatment. In 2006, the FDA also approved a labeling extension for bevacizumab (Avastin, Genentech, Inc.), when administered in combination with carboplatin and paclitaxel, for the initial systemic treatment of patients with unresectable, locally advanced, recurrent, or metastatic, nonsquamous, non-small cell lung cancer.
We are repeatedly told that these drugs lead to a statistically significant improvement in survival, usually as demonstrated through one or two randomized controlled trials. What we are less often told is that the overall survival benefit of these drugs is in the realm of 6 to 8 weeks! That is why they have so little impact on the broader survival trends that are reflected in the above charts. They hardly do anything in terms of real-life situations. Crudely put, the patient trades some serious toxicity for 1 to 2 months of increased survival.
Let us look at just one such clinical trial to get a glimpse into the actual survival numbers. This was E4459, a clinical trial initiated by the Eastern Cooperative Oncology Group (ECOG) and published in the Journal of Clinical Oncology in 2005. Treatment involved the familiar doublet of paclitaxel and carboplatin (abbreviated as PC) vs. a cohort that also received bevacizumab (abbreviated PCB). The trial looked at patients with previously untreated patients with stage IIIb (involving pleural effusion) or stage IV non-small cell lung cancer. The trial's primary endpoint was overall survival. Secondary endpoints were response rate, time to progression and tolerability of the drugs.
The response rate was much higher in the group that received Avastin (bevacizumab). They had tumor shrinkage in 27%, compared with just 10% of the PC group. However, the difference in progression-free survival was much less, 4.5 months for PC vs. 6.4 months for PCB. Finally, the impact on overall survival was a little over two months. Median overall survival was 10.2 months with PC vs. 12.5 months with Avastin-added, or a benefit of 2.3 months. According to the authors, PCB thereby became "ECOG's new treatment standard in this patient population."
However, here's how it looks from the patient's perspective. The three drugs in combination did extend survival by about 2 months. Meanwhile, "grade 4/5 neutropenia" occurred in 24% of the patients receiving the triplet (and there were other serious side effects as well). Grade 4 neutropenia involves life-threatening consequences, with urgent intervention indicated. And what exactly is "grade 5 neutropenia"? There's another more common term for this: death.
So for an increased survival of about 2 months (or at least such was the case in the rarefied atmosphere of a clinical trial), the patient faced a 25% chance of coming close to, or actually, dying of the treatment itself. I will take it as axiomatic that the chances of responding go down, and the chances of having serious or life-threatening side effects, go up when the drugs in question are transferred to the community setting.
I know that most oncologists believe that they are serving their patients well by giving them these drugs, even if some oncologists would not take them themselves if they had lung cancer. But their thinking on the treatment of lung cancer still largely takes place within parameters set by the pharmaceutical industry. In my opinion, the limited choice offered to American cancer patients is more about the $6 billion in annual sales of Avastin than actual patient benefit. As I have said before, doctors who truly care about their patients would do better to take a serious look at what is available in the field of complementary and alternative medicine (CAM), and advocate forcefully for clinical trials to further test the potential of the most promising less-toxic treatments.

References
CTCAE4 Blood and lymphatic system disorders:Febrile neutropenia [Web page]. BiomedGT Collaborative Ontology Development Wiki. http://biomedgt.nci.nih.gov/wiki/index.php/CTCAE4_
Blood_and_lymphatic_system_disorders:Febrile_neutropenia. (One link on two lines.)
National Cancer Institute: Common Terminology Criteria for Adverse Events. v3.0 (CTCAE). Washington, DC; 2003.
American Society of Clinical Oncology/FDA Lung Cancer Endpoints Workshop (April 15, 2003).

Ralph W. Moss, PhD, is the author of 12 books on cancer-related topics. The former science writer at Memorial Sloan-Kettering Cancer Center, for 35 years Moss has investigated the validity of many cancer treatments. He currently directs the Moss Reports, a library of reports for patients on over 200 different cancer diagnoses.
|



![]()
![]()
![]()




