| Online
publication only
Biochemistry
Iron is one of the most abundant earth elements, yet only traces
are essential for living cells of plants and animals. In humans,
most of the iron is contained within the porphyrin ring of heme
in proteins such as hemoglobin, myoglobin, catalase, peroxidases,
and cytochromes. as well as iron-sulfur proteins such as NADH dehydrogenase
and succinate dehydrogenase, in which iron is present in clusters
with inorganic sulfur. In all these systems, iron has the ability
to interact reversibly with oxygen and to function in election transfer
reactions that makes it biologically indispensable.
Pathophysiology
The average adult male contains approximately 4 grams of body iron.
About 65% to 70% is found in hemoglobin, 4% in myoglobin, and less
than 1% in other iron-containing enzymes and proteins. The remaining
25% to 30% represent the storage pool of iron. By contrast, women
have a much smaller iron reserve, with the adult female body containing
about 3 grams of iron. Women also have a slightly lower hemoglobin
concentration in blood than males. Patients with iron overload diseases
may store as much as 20 g of iron.
Excess iron can result in cell injury. Menstruation, bleeding due
to injury, or bloodletting help to excrete excess iron. Other than
that, humans do not excrete excess iron effectively.
Iron overload can result from an increased absorption of dietary
iron or from parenteral administration of iron. When the iron burden
exceeds the body's capacity for safe storage, the result is widespread
damage to the liver, heart, joints, pancreas, and other endocrine
organs.1 It must be noted that low serum iron alone is not an indicator
of iron deficiency. Serum ferritin, transferrin levels and total
iron binding capacity must confirm the diagnosis before iron is
supplemented. To improve iron absorption and utilization, adequate
amounts of vitamins C and B, especially folic acid, B6, and B12,
must be provided.
Diseases of Iron
Overload
There are several inherited and acquired disorders such as hemochromatosis
that can result in chronic iron overload in humans. The major clinical
consequences are hepatic fibrosis, cirrhosis, hepatocellular cancer,
cardiac disease, and diabetes. Lipid peroxidation is a result of
iron overload.
Researchers from the Department of Internal Medicine, St. Louis
University Health Sciences Center, Missouri, write: "In the
liver, this lipid peroxidation is associated with impairment of
membrane-dependent functions of mitochondria and lysosomes. Iron
overload impairs hepatic mitochondrial respiration primarily through
a decrease in cytochrome C oxidase activity, and hepatocellular
calcium homeostasis may be compromised through damage to mitochondrial
and microsomal calcium sequestration. DNA has also been reported
to be a target of iron-induced damage, and this may have consequences
in regard to malignant transformation."2,3
Iron overload or acquired hemochromatosis may be a complication
of chronic anemias such as thalassemia and sideroblastic anemia.
In the initial stage of iron overload, referred to as hemosiderosis,
tissues remain anatomically and functionally normal. As the iron
load increases, the clinical pattern resembles that of hemochromatosis.
Acquired hemochromatosis can result from frequent blood transfusions
or excess iron ingestion. The Bantu of South Africa drink home-brewed
alcoholic beverages with a very high iron content and therefore
have a high incidence of the disease. Alcoholics with chronic liver
disease such as cirrhosis are also susceptible to developing an
increase in iron storage. Genetic predisposition increases the onset
of the disease.
Signs and symptoms of hemochromatosis are related to the organs
involved, such as:
· liver enlargement and cirrhosis;
· hepatocellular carcinoma;
· diabetes mellitus (in about two-thirds of the patients);
· increase in skin pigmentation, caused by increased melanin
production, found in 90% of patients;
· cardiac involvement, which may lead to congestive heart
failure or arrhythmia;
· testicular atrophy;
· loss of libido, caused by drop in production of gonadotropins
by the impaired hypothalamus-pituitary axis.
Sources of excess iron:
· iron-rich drinking water;
· cooking acidic food in iron cookware;
· excessive iron supplementation or prolonged iron therapy;
· repeated blood transfusions;
· protein malnutrition.
Therapeutic considerations:
· Phlebotomy therapy is treatment of choice.
· Iron-chelation with Deferoxamine or other iron-specific
chelating agents
· Vegetarian diet
· Check blood for manganese, copper, and zinc levels, and
balance blood chemistry.
· Vitamin C must be restricted. Doses should be not higher
than 250–500mg/day and should be used only on those days when
Desferal infusion or other iron chelation is performed.6
Diagnosing Hemochromatosis
To diagnose the disease is not difficult. There are basically three
tests that confirm an iron overload.
Test 1. Transferrin Saturation (TS) or,
as it is called in some labs, percentage of saturation: After a
12-hour fast, measure total iron binding capacity (TIBC) and the
serum iron (SI). To achieve the percentage of saturation you divide
the TIBC into SI.
Serum Iron SI
------- = Yields Transferrin Saturation (TS)
Total Iron Binding TIBC or in some labs percentage of saturation
Capacity
Safe range = 12–44%
Any values above this range must be considered
diagnostic for hemochromatosis and should cause immediate treatment.
Any values far below this range may be a sign of bleeding ulcers,
chronic infection, or cancer. Physicians should look for the cause
of anemia.
Test 2. Serum Ferritin (SF): Using the blood from the first draw,
next check the amount of storage iron.
Safe range = 5–150
A hemochromatosis patient needs to be at
the lowest end of this range. Below 10 is the treatment goal.
Test 3. Unbound iron binding capacity
(UIBC), used less frequently.
Safe range is above 146
If a patient checks below this test value,
then he or she needs to be treated for hemochromatosis or other
iron overload conditions.
How Common Are
Iron Overload Diseases?
One in three Irish natives is predisposed genetically. In the US,
a carrier rate of 20% is reported for Irish Americans. African Americans
too have a 20% carrier rate in the US. Typical for this population
is that the main screening lab value – transferrin saturation
(TS) – sometimes seems normal. To evaluate this group, more
attention must be paid to family history, symptoms, and a complete
diagnostic schedule as outlined above.
People with hemochromatosis should not take iron or vitamin C supplements,
which increase iron availability. Those who have liver damage should
not consume alcoholic beverages or eat raw seafood.
Treating Iron Overload
The object is to induce a mild anemia and maintain it until the
storage iron is greatly reduced. Serum ferritin is the measure of
storage iron, and it needs to come down below 10. This is accomplished
by therapeutic phlebotomies, which must be done regularly in a medical
setting. The treatment schedule may continue for 6 months to 3 years,
depending on the iron burden. Age is never a reason to disqualify
someone from treatment. Frailty, small stature, and extreme youth/age
may require adjustments in the amount of blood removed, but never
the frequency. The process of bloodletting can arrest or reverse
most symptoms and return the patient to a normal life span. Some
patients might experience a complete reversal of all symptoms. Unfortunately,
treatment cannot cure the conditions associated with established
hemochromatosis, but it will bring relief to most. The main exception
is arthritis, which is said not to improve even after excess iron
is removed. To exclude any hemochromatosis patient from treatment
for any reason is a death sentence.
Chelation
Chelation can be an alternative when bloodletting is contraindicated.
There are three agents used for iron chelation in the US: Exjade,
Desferal (also called Deferoxamin or desferrioxamine), and Deferiprone,
usually limited to thalassemia major.
Desferal can be given intravenously or subcutaneously. It is rapidly
absorbed after administration, but only poorly absorbed from the
gastrointestinal tract in the presence of intact mucosa. Recommended
are infusions of 2 or 2.5 grams; 100 mg is capable of binding approximately
8.5 mg of trivalent (ferric) iron.
To evaluate the efficacy of Desferal therapy, a 24-hour urine collection
should be performed on the third day for iron determination. This
value should be compared with a preinfusion 24-hour urine iron determination.
In order to gain benefit from the use of Desferal, a 24-hour excretion
of 30 mg or more of iron is generally required, particularly for
patients with significant ongoing transfusion requirements. Patients
should be warned that their urine will become orange. This is the
iron-Desferal complex (ferrioxamine), which is being excreted.
For some hemochromatosis patients, Desferal is infused overnight
with a portable pump at home during sleep over a 12-hour period.
In some cases, the infusion pump is installed in the body of the
patient.4 Monthly Desferal treatments may cost $6000 to $8000.
Exjade and Deferiprone are similar in action to Desferal, are used
orally, have fewer side effects, and are less expensive.5
Ethylenediamine
Tetraacetic Acid (EDTA)
EDTA has a good iron binding capacity, and while intravenous EDTA
chelation is not mentioned in any conventional medical textbook,
it does provide options for mild cases of iron overload or as a
maintenance schedule for hemochromatosis. The treatment costs would
be comparatively inexpensive. A treatment plan, consisting of one
infusion per week, would cost $600 to $1,000 per month. Interestingly,
alternative medicine has not yet recognized nor studied the beneficial
effects of EDTA's iron-binding ability. Controlled studies
are needed.
The following chart reflects EDTA's iron-binding capacity
in comparison to DMPS (2,3-Dimercapto-1-propanesulfonic acid) and
DMSA (meso-2,3-Dimercaptosuccinic acid). Test values represent a
95th percentile and have been obtained from people with normal iron
status. It can be assumed that EDTA chelation performed on a hemochromatosis
patient would result in a greater iron binding than seen in Table
1. The 95th-percentile level of about 800 mcg/g creatinine (= approx
0.8mg/L) obtained from a pool of people who do not have an iron-overload
problem. It explains the anti-inflammatory benefit of EDTA chelation
treatment.
Table 1
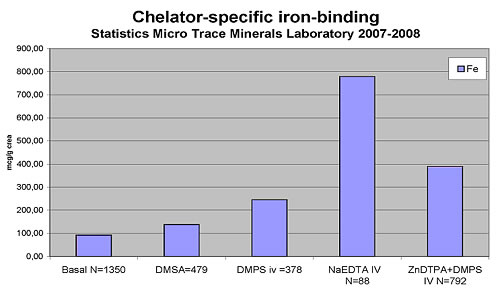
Maintenance
After the patient has had his ferritin reduced below 10, he is declared
deironed. Now it is time to change the phlebotomy schedule, and
this is often a matter of trial and error by the physician and patient.
Serum ferritin should be checked regularly, at least yearly. Maintenance
will have to be a lifetime affair. To permit iron to reaccumulate
is to invite premature death.

Dr. E. Blaurock-Busch is
laboratory and research director at Micro Trace Minerals Clinical
Laboratory of Germany and Boulder, Colorado. She is scientific advisor
to the IBCMT (International Board of Clinical Metal Toxicology)
and the German Medical Association for Clinical Metal Toxicology.
She has written books on laboratory diagnostics and chelation.
For more information, contact
the author at info@microtraceminerals.com.

1. Britton RS, Bacon BR, Recknagel RO.
Lipid peroxidation and associated hepatic organelle dysfunction
in iron overload. Chem Phys Lipids.
1987 Nov-Dec;45(2-4):207-239.
2. Britton RS, Ramm GA, Olynyk J, Singh R, O'Neill R, Bacon BR.
Pathophysiology of iron toxicity. Adv Exp
Med Biol. 1994;356:239-253.
3. Britton RS, Leicester KL, Bacon BR. Iron toxicity and chelation
therapy. Int J Hematol. 2002 Oct;76(3):219-228.
4. Porter JB. A risk-benefit assessment of iron-chelation therapy.
Drug Saf. 1997 Dec;17(6):407-421.
5. Maggio A, D'Amico G, Morabito A, et al. Deferiprone versus deferoxamine
in patients with thalassemia major: a randomized clinical trial.
Blood Cells Mol Dis. 2002 Mar-Apr;28(2):196-208.
6. Information Center for Sickle Cell and Thalassemic Disorders.
Subcutaneous Desferal® for transfusional iron overload. Available
at: http://sickle.bwh.harvard.edu/dfsc.html.
|



![]()
![]()
![]()


