Page 1, 2
There are two additional comparisons that need to be discussed from these data. Total biopterin in plasma is composed primarily of BH4 and BH2, with relatively little of the fully oxidized product, biopterin, being present because biopterin is fairly rapidly excreted into the urine. That is why the biopterin measurements reported here are all urine measurements. It follows from this that total biopterin, as shown in Table 2, can be compared with BH4 plus BH2 levels shown in Table 1. Such a comparison shows that all of the BH4 plus BH2 values for the 17 patients before sauna therapy are within the normal reference range, showing that BH4 biosynthesis is normal and that the low BH4 levels are caused by BH4 oxidation (presumably by peroxynitrite). This is confirmed by the observation that 6 out of 17 patients have BH4/BH2 ratios below the reference range. Surprisingly, however, 2 out of 17 patients have BH4 /BH2 ratios above the reference range. When one studies the BH4 + BH2 values after sauna treatment, there is a great increase, such that 12 of 17 patients are above the reference range and only patients 8, 10, 13, 15, and 17 are within the reference range and these are all in the upper half of that range (compare Table 1 and 2). Thus there is a striking increase in BH4 synthesis following sauna therapy, leading to a striking increase in total biopterin.
Figure 1: NO/ONOO− Diagram
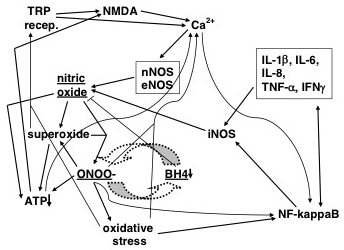
Each arrow represents one or more mechanisms by which one element of the cycle increases another. The various elements include nitric oxide (NO), central upper left; superoxide, central left; peroxynitrite (ONOO−), central lower left; oxidative stress (an imbalance between oxidants and antioxidants), bottom central; NF-kappaB, an important transcription factor that stimulates transcription of many genes (lower right corner), including the inflammatory cytokines (upper right box) and iNOS, central, slightly right; intracellular calcium (Ca2+), top, a cascade of events leading to mitochondrial dysfunction and lowered APTY pools (lower left corner); excessive activity of the NMDA receptors (top, left central); lowered levels of tetrahydrobiopterin (BH4, center slightly right); and increased activity of several of the TRP family of receptors. The two arrows most relevant to this study are the dashed arrows linking peroxynitrite (ONOO−) with BH4 depletion. The right-pointing arrow mechanism is extensively documented and involves peroxynitrite oxidation of BH4; the left-pointing arrow must be viewed, however, as somewhat speculative; and it is important, therefore, that this study provides some evidence supporting this arrow.
The most surprising result seen in Table 1 is that the elevated neopterin levels in ME/CFS patients are greatly reduced following sauna therapy. An understanding of the mechanism of neopterin generation is needed here in order to interpret these results. Under inflammatory conditions, several inflammatory cytokines induce both the inducible nitric oxide synthase (iNOS; see Figure 1) and also GPCH, with the GPCH induction providing the increased BH4 needed to prevent widespread uncoupling of the iNOS enzyme. Such widespread uncoupling would lead to the production of large amounts of superoxide by that enzyme.7 However, in primates, there is little induction of the second enzyme in the pathway, pyruvoyl tetrahydropterin synthase (PTPS). This leads to accumulation of the second intermediate in the pathway, dihydroneopterin triphosphate, which is degraded to neopterin which is then excreted in the urine.23-25 It is surprising, therefore, that sauna therapy which we show leads to increased levels of GPCH also leads to lowered neopterin rather than raised neopterin. There is only one logical explanation for this finding, sauna therapy must act to produce a large increase in the second enzyme in the pathway, PTPS, as well, leading to increased metabolism of the dihydroneopterin triphosphate in the BH4 biosynthetic pathway and therefore decreased generation of neopterin. So the question is whether there is a mechanism by which sauna therapy can lead to increased PTPS. The answer is that there is.
The cytokine IL-1b has been shown to induce PTPS but not GPCH, unlike other cytokines; and the action of IL-1b has been shown to be greatly stimulated by the heat shock protein Hsp90, which is induced by the heat of sauna therapy.26-29 The mechanism of stimulation of IL-1b action is through the stimulation of the action of its receptor.30 It should be noted that we have no independent evidence that this is the mechanism of sauna therapy in raising PTPS activity, but it would be surprising if there were a second such mechanism by which sauna treatment raises PTPS activity. In any case, the raising of PTPS activity provides a second mechanism by which sauna therapy raises BH4 synthesis, since lowering the degradative loss of neopterin triphosphate in the BH4 biosynthetic pathway will, of course, lead to increased biosynthesis of BH4.

Notes
1. Pall ML. Do sauna therapy and exercise act by raising the availability of tetrahydrobiopterin? Med Hypotheses. 2009;73:610–613.
2. Pall ML. Pulmonary hypertension is a probable NO/ONOO− cycle disease: A review. ISRN Hypertens. 2013. Article ID 742418.
3. Pall ML. Explaining "Unexplained Illness": Disease Paradigm for Chronic Fatigue Syndrome, Multiple Chemical Sensitivity, Fibromyalgia, Post-Traumatic Stress Disorder, Gulf War Syndrome and Others. New York: Harrington Park (Haworth) Press; 2007.
4. Pall ML. The NO/ONOO− vicious cycle mechanism as the cause of chronic fatigue syndrome/myalgic encephalomyelitis. In: Svoboda E, Zelenjcik K, eds. Chronic Fatigue Syndrome: Symptoms, Causes and Prevention. Nova Publishers; 2010:27–56.
5. Pall ML. Elevated, sustained peroxynitrite levels as the cause of chronic fatigue syndrome. Med Hypotheses. 2000;54:115–125.
6. Pall ML. Multiple chemical sensitivity: Toxicological questions and mechanisms. In: General and Applied Toxicology. 3rd ed. John Wiley & Sons; 2009:2303–2352.
7. Pall ML. Nitric oxide synthase partial uncoupling as a key switching mechanism for the NO/ONOO− cycle. Med Hypotheses. 2007;69:821–825.
8. Gibson PR, Elms AN-M, Ruding LA. Perceived treatment efficacy for conventional and alternative therapies reported by persons with multiple chemical sensitivity. Environ Health Perspect. 2003;111:1498–1504.
9. Rea WJ. Chemical Sensitivity. Boca Raton, FL: Lewis Publishers; 1992.
10. Crinnion W. Components of practical clinical detox programs – sauna as a therapeutic tool. Altern Ther Health Med. 2007;13:S154–S156.
11. Baird DN, Rea WJ. The temperomandibular joint implant controversy. Part II: its clinical implications. J Nutr Environ Med. 1999;9:209–222.
12. Rogers SA. Detoxify or Die. Syracuse, NY: Prestige Publishers; 2002.
13. Krop J. Chemical sensitivity after intoxication at work with solvents: response to sauna therapy. J Altern Complement Med. 1998;4:77–86.
14. Genuis SJ, Birkhloz D, Rodushkin I, Beesoon S. 2011 Blood, urine, sweat (BUS) study: monitoring and eliminating bioaccumulated toxic elements. Arch Environ Contam Toxicol. 61:344–357.
15. Cecchini M, LoPresti V. Drug residues store in the body following cessation of use: impacts on neuroendocrine balance and behavior – use of Hubbard sauna regimen to remove toxins and restore health. Med Hypotheses. 2007;68:868–879.
16. Cecchini MA, Root DE, Rachunow JR, Gelb PM. Chemical exposures at the World Trade Center: use of Hubbard detoxification regimen to improve health status of New York City rescue workers exposed to toxicants. Townsend Lett. 2006;273:58–65.
17. Ross GH, Sternquist MC. Methamphetamine exposure and chronic illness in police officers: significant improvement with sauna-based detoxification therapy. Toxicol Ind Health. 2012;28:758–768.
18. Parpale IA, Prokof'eva LG, Obertas VG. The use of the sauna for disease prevention in the workers of enterprises with chemical and physical occupational hazards. [Article in Russian.] Vrach Delo. 1991 May;(5):93–95.
19. Fukuda et al. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann Intern Med. 1994;121:953–959.
20. Cai S, Alp, NJ, McDonald D, et al. GTP cyclohydrolase I gene transfer augments intracellular tetrahydrobiopterin in human endothelial cells: effects on nitric oxide synthase activity, protein levels and dimerisation. Cardiovasc Res. 2002;55;838–849.
21. Crowley JR, Yarasheski K, Leeuwenburgh C, Turk J, Heinecke JW. Isotope dilution mass spectrometric quantification of 3-nitrotyrosine in proteins and tissues is facilitated by reduction to 3-aminotyrosine. Anal Biochem. 1998;259:127–135.
22. Schwedhelm E, Tsikas D, Gurzke FM Frolich J. Gas chromatographic-tandem mass spectrometric quantification of free 3-nitrotyrosine in human plasma at the basal state. Anal Biochem. 1999;276:195–203.
23. Werner ER, Werner-Felmayer G, Fuchs D, et al. Tetrahydrobiopterin biosynthetic activities in human macrophages, fibroblasts, THP-1, and T 24 cells. GTP-cyclohydrolase I is stimulated by interferon-gamma, and 6-pyruvoyl tetrahydropterin synthase and sepiapterin reductase are constitutively present. J Biol Chem. 1990;265:3189–3192.
24. Werner ER, Werner-Felmayer G, Wachter H. Tetrahydrobiopterin and cytokines. Proc Soc Exp Biol Med. 1993;203:1–12.
25. Wachter H, Fuchs D, Hausen A, et al. Neopterin Biochemistry –Methods – Clinical Application. Berlin: Walter De Gruyter; 1992.
26. Franscini N, Blau N, Walter RB, Schaffner A, Schoeden G. Critical role of interleukin-1beta for transcriptional regulation of endothelial 6-pyruvoyltetrahydropterin synthase. Anterioscler Thromb Vasc Biol. 2003;23:e50–e53.
27. Shi L, Zhang , Fang S, et al. Heat shock protein 90 (Hsp90) regulates the stability of transforming growth factor beta-activated kinase 1 (TAK1) in interleukin-1beta-induced cell signalling. Mol Immunol. 2009;46:541–550.
28. Rice JW, Veal JM, Fadden RP, et al. 2008 Small molecule inhibitors of Hsp90 potently affect inflammatory disease pathways and exhibit activity in models of rheumatoid arthritis. Arthritis Rheum. 58:3765–3775.
29. Lukart SD, Panoskaltis-Mortari A, Hinkel T, et al. Mactinin, a fragment of cytoskeletal alpha-actinin, is a novel inducer of heat shock protein (Hsp-90) mediated monocyte activation. BMC Cell Biol. 2009 Aug 28;10:60.
30. De Nardo D, Masendycz P, Ho S, et al. A central role for the Hip90-Cdc37 molecular chaperone module in Interleukin-1 receptor-associated-kinase-dependent signalling by toll-like receptors. J Biol Chem. 2005;280:9813–9822.
Correspondence:
Martin L. Pall, PhD
638 NE 41st Ave.
Portland, Oregon 97232-3312
martin_pall@wsu.edu
thetenthparadigm.org
 Tapan Audhya, PhD, received his doctorate degree in biochemistry and chemistry of natural products from Dalhousie University, Canada. Prior to his recent responsibility as director of research and development at Health Diagnostics & Research Institute, he spent 12 years in research and development of various divisions of the pharmaceutical industry. He is also associated with New York University Medical School as a research professor. With over 190 published articles in refereed journals and books, Dr. Audhya is an expert oan endocrinology, toxicology, metabolism, and nutritional biochemistry. For the last 15 years, he has been involved in autism, chronic fatigue syndrome, and depression and presented many of his findings at international and national conferences. Tapan Audhya, PhD, received his doctorate degree in biochemistry and chemistry of natural products from Dalhousie University, Canada. Prior to his recent responsibility as director of research and development at Health Diagnostics & Research Institute, he spent 12 years in research and development of various divisions of the pharmaceutical industry. He is also associated with New York University Medical School as a research professor. With over 190 published articles in refereed journals and books, Dr. Audhya is an expert oan endocrinology, toxicology, metabolism, and nutritional biochemistry. For the last 15 years, he has been involved in autism, chronic fatigue syndrome, and depression and presented many of his findings at international and national conferences.

Martin L. Pall, PhD, received his BA degree from Johns Hopkins University and his PhD from California Institute of Technology. He is professor emeritus of biochemistry and basic medical sciences at Washington State University. He has received eight international honors for his work in disease mechanisms and related issues.
 John A Green III, MD, earned his MD at the University of Utah. He was an emergency department physician from 1975 to 1980, and emergency department director from 1978 to 1980 at Tulare District Hospital (Tulare, CA). He had a holistic/environmental/orthomolecular medicine practice from 1980 to 1999. Since then, his primary focus has been on autism research, education, and treatment: providing diagnosis and treatment to approximately 2500 children and adults with autistic spectrum disorders. Dr. Green is the author of A Diagnosis for Man (Two Rivers Press; 1985) and "Chemical Sensitivity and the Scientific Method" (J Pestic Reform. 1989), and contributed two chapters to Treating Autism (Rimland B, Edelson S, eds. 2003). John A Green III, MD, earned his MD at the University of Utah. He was an emergency department physician from 1975 to 1980, and emergency department director from 1978 to 1980 at Tulare District Hospital (Tulare, CA). He had a holistic/environmental/orthomolecular medicine practice from 1980 to 1999. Since then, his primary focus has been on autism research, education, and treatment: providing diagnosis and treatment to approximately 2500 children and adults with autistic spectrum disorders. Dr. Green is the author of A Diagnosis for Man (Two Rivers Press; 1985) and "Chemical Sensitivity and the Scientific Method" (J Pestic Reform. 1989), and contributed two chapters to Treating Autism (Rimland B, Edelson S, eds. 2003).
Page 1, 2 |
![]()
![]()
![]()






 Tapan Audhya, PhD, received his doctorate degree in biochemistry and chemistry of natural products from Dalhousie University, Canada. Prior to his recent responsibility as director of research and development at Health Diagnostics & Research Institute, he spent 12 years in research and development of various divisions of the pharmaceutical industry. He is also associated with New York University Medical School as a research professor. With over 190 published articles in refereed journals and books, Dr. Audhya is an expert oan endocrinology, toxicology, metabolism, and nutritional biochemistry. For the last 15 years, he has been involved in autism, chronic fatigue syndrome, and depression and presented many of his findings at international and national conferences.
Tapan Audhya, PhD, received his doctorate degree in biochemistry and chemistry of natural products from Dalhousie University, Canada. Prior to his recent responsibility as director of research and development at Health Diagnostics & Research Institute, he spent 12 years in research and development of various divisions of the pharmaceutical industry. He is also associated with New York University Medical School as a research professor. With over 190 published articles in refereed journals and books, Dr. Audhya is an expert oan endocrinology, toxicology, metabolism, and nutritional biochemistry. For the last 15 years, he has been involved in autism, chronic fatigue syndrome, and depression and presented many of his findings at international and national conferences.
 John A Green III, MD, earned his MD at the University of Utah. He was an emergency department physician from 1975 to 1980, and emergency department director from 1978 to 1980 at Tulare District Hospital (Tulare, CA). He had a holistic/environmental/orthomolecular medicine practice from 1980 to 1999. Since then, his primary focus has been on autism research, education, and treatment: providing diagnosis and treatment to approximately 2500 children and adults with autistic spectrum disorders. Dr. Green is the author of
John A Green III, MD, earned his MD at the University of Utah. He was an emergency department physician from 1975 to 1980, and emergency department director from 1978 to 1980 at Tulare District Hospital (Tulare, CA). He had a holistic/environmental/orthomolecular medicine practice from 1980 to 1999. Since then, his primary focus has been on autism research, education, and treatment: providing diagnosis and treatment to approximately 2500 children and adults with autistic spectrum disorders. Dr. Green is the author of