Abstract
Objective: To
simultaneously assess the rate of aerobic bacteria, yeast-mold proliferation
count in 2 red delicious apple sections from a parent
apple specimen, with and without proximal exposure to a packet of
Zeolite-coated Potassium Permanganate (Z-KMnO4). (Note: This
is a plastic louvered container enclosed sachet filled with Potassium
Permanganate,
KMnO4.)
Design, Sample, Location: Following
28-days' refrigeration in
separate compartments, two "Red Delicious" apple samples
excised from a single specimen were reduced to 1:1000 solutions then
incubated for 36 and 72 hours to determine aerobic bacteria and yeast-mold
counts respectively, in Northeastern Washington. One sample was exposed
to an antimicrobial mineral compound, Z-KMnO4, while the other sample
was isolated (compartment-separated) from the antimicrobial Z-KMnO4
mineral compound.
Results: Harvested apples predictably release ethylene gas, which acts
as a ripening hormone. As ripening progresses, commensal environmental
microbes proliferate in samples exposed to air. Eating over-ripened,
microbe-infected spoiled produce has been associated with severe gastric
stress, compromised immunity, and allergic reactions. The spoilage
rate of stored produce is dependent upon time, temperature, viscosity,
pH, humidity, space, and nutrient access. Refrigeration in temperatures
below 50 degrees F and under 45% humidity delays the rate at which
produce deteriorates by inhibiting ethylene gas ripening hormone release,
hence the rate at which aerobic bacteria, yeast, and mold grows in
produce. Zeolite-coated Potassium Permanganate (Z-KMnO4) placed proximal
to refrigerated produce reduces ripening rate, inhibits microbial growth,
and spoilage. Identifying which microbes and how many are inactivated
by packets of Z-KMnO4 in refrigerator compartments, until now, has
not been identified. A randomly selected Red Delicious apple sample
was refrigerated for 28-days and enclosed adjacent to a single Z-KMnO4
packet. This sample, following incubation, yielded 10,000 aerobic bacteria
per milliliter count. Simultaneously, an apple section from the same
apple was refrigerated for 28-days without Z-KMnO4 exposure. This sample
yielded a count of 10,000,000 aerobic bacteria per milliliter. From
the same Red Delicious apple sample, both sections after being refrigerated
for 28-days surprisingly yielded an equal ratio of 100,000 yeast/mold
per milliliter in the sample with Z-KMnO4, and 100,000 yeast/mold per
milliliter in the sample isolated from exposure to Z-KMnO4.
Conclusion: This shows that a single packet (Z-KMnO4) stored with a
refrigerated apple sample reduced the aerobic bacteria (AB) proliferation
growth count by a factor of 1000, (10,000 AB Z-KMnO4) as compared to
10,000,000 AB non-exposed sample). However, Z-KMnO4 exposure did not
reduce yeast/mold growth in either sample (100,000 Z-KMnO4 as compared
to 100,000 in non-exposed sample). Given the cost to health from incidental
consumption of spoiled produce, reducing the rate of aerobic bacteria
growth in produce is a nutrient-protective intervention with disease-preventative
implications. This single experimental study is conclusively limited
and therefore requires more extensive research in order to explain
or confirm the evidence reported.

Introduction
Ethylene Gas (C2H4), a hydrocarbon, is a natural product of plant metabolism
and is produced by all tissues of plants as a hormone, increasing
the rate of aging and ripening properties. Harvested apples
release significant amounts of ethylene,
a hormone-ripening agent. During the ripening stage of fruit,
ethylene gas accelerates the maturity
process. Ethylene gas (released at a very high rate from
apples)
accelerates ripening, loss of chlorophyll, abortion of plant
parts, stem shortening, abscission of plant parts, and epinasty
(Han JH,
2003).

Apple Rate of Ethylene Production
| Temperature |
0°C (32°F) |
5°C (41°F) |
10°C (50°F) |
20°C (68°F) |
| µ 1/ kg·hr |
1–10 |
2–20 |
5–40 |
20 –125 |
Higher rates for riper apples.
From: Postharvest Technology Research and Information Center, Department
of Pomology; University of California, One Shields Ave., Davis, CA
95616-8683.
By permission, courtesy of Dave Biswell, President, Ethylene Control
Inc.
(559) 896-1909; (800) 200-1909; www.ethylenecontrol.com
Apple 'Red Delicious' Recommendations for Maintaining Postharvest Quality
http://www.ethylenecontrol.com/technical/uc105.htm 
Table V.
How Ethylene Gas Affects Produce
(Ethylene production and sensitivity levels in selected fresh produce)
Fruits & Vegetables
Types
|
Rate of Ethylene |
Ethylene Sensitivity |
Principal Reaction to Ethylene Gas |
APPLES
Apricots
Asian Pears
Asparagus
Avocados
Bananas
Berries
Broccoli
Brussel sprouts
Cantaloupe
Carrots
Cherimoya
Cherries
Cucumbers
Eggplant
Grapefruit
Grapes
Kiwifruit
Lemons, Limes
Lettuce (2)
Mangoes
Melons (3)
Nectarines
Onions, Garlic
Oranges
Papaya
Passion Fruit
Peaches
Pears (5)
Persimmons
Plums, Prunes
Potatoes (6)
Quinces
Tomatoes
Watermelons |
VH
H
H
VL
H
M
L
VL
VL
H
VL
VH
VL
L
L
VL
VL
L
VL
VL
M
M
H
VL
VL
H
VH
H
H
L
M
VL
L
M
L
|
H
H
H
M
H
H
L
H
H
M
L
H
L
H
M-H
M
L
H
M
H
H
H
H
L
M
H
H
H
H
H
H
M
H
H
H |
SCALD(1)
Decay
Decay
Toughness
Decay
Decay
Mold
Yellowing
Yellowing
Decay
Bitterness
Decay
Softening
Yellowing
Brown
Spots
Mold
Mold
Decay
Mold
Russet spotting
Decay
Decay
Decay
Odor, sprouting
Mold
(4)
Decay
Decay
Decay
Decay
Decay
Decay
Sprouting
Decay
Shrink, decay
Lose
firmness |
Table V Notes:
VL
= Very low
L = Low
M = Moderate
H = High
VH = Very High
(1) Lose
crunch
(2)
Leafy greens
(3) Crenshaw, Honeydew, Persian
(4) Rind breakdown
(5)
Anjou,
Bartlett, Bosc
(6) Processing, Seed
Fresh Produce Manual for
1997 from
the Produce Marketing Association and the 1991 Sea Land Shipping
Guide
for Perishables. By permission, courtesy
of Dave
Biswell, President, Ethylene Control Inc. 559-896-1909; 800-200-1909; www.ethylenecontrol.com 
Following harvest, apple produce commences to release ripening-hormone
ethylene gas. Over-ripening reduces the shelf life of apples, and so
the reduction of ethylene is necessary. Ethylene gas removers include
potassium permanganate (KMnO4), zeolite, clay, bentolite, alumino-silicate
and active carbon. Other sources of ethylene include ripening fruit,
rotting vegetation, exhaust from internal combustion engines/heaters,
smoke (including cigarettes), welding, and natural gas leaks (Han JH,
2003).
As fruit ripens, the fluid-juice within the fruit body supports aerobic
bacteria and yeast/mold proliferation. Food-borne illnesses may occur
due to incidental consumption of commercial, nonpasteurized ("fresh" or "unpasteurized")
fruit juices (Matthys AW). Nonpasteurized fruit juice has been associated
with numerous food-borne illness outbreaks since the 1920s. Disease
syndromes have included salmonellosis, typhoid fever, cryptosporidiosis,
Escherichia coli-related diarrhea, and hemolytic uremia (Parish 1997).

Apple Juice-associated Food Poisoning Outbreaks
| Juice Product (Year) |
Infectious Agent |
Sweet cider (1923)
Apple cider (1974)
Apple cider (1980)
Apple cider (1991)
Apple cider (1993)
Apple cider (1993)
Apple juice (1996)
Apple juice (1996)
Apple juice (1996)
Apple cider (1997)
Apple cider (1998)
Apple cider (1999)
|
Salmonella typhi
S. typhimurium
Enterotoxigenic E. coli
E.coli 0157:H7
E.coli 0157:H7
Cryptosporidium spp
E.coli 0157:H7
E.coli 0157:H7
Cryptosporidium parvum
E.coli 0157
E. coli 0157:H7
E. coli 0157:H7 |
Table Notes: Parish ME.1997. Public
health and nonpasteurized fruit juices. Crit
Rev Microbiol 23(2):109-19; Bates R.P.,
Morris J.R., Crandall P.G. Principles and practices of small - and medium -
scale fruit juice processing, FAO Agricultural
Services Bulletin 146. FAO 2001. (See
Chapter 4 table.) Beuchat LR, Nail BV, Adler BB, Clavero MR. Efficacy of spray
application of chlorinated water in killing pathogenic bacteria on raw apples,
tomatoes, and lettuce. J
Food Prot. 1998 Oct;61(10):1305-11. 
Aerobic bacteria, E. coli 0157:H7, L. monocytogenes, and Salmonella proliferate
in apple juice. Escherichia coli 0157:H7, Listeria monocytogenes, and
Salmonella have been detected in samples of apple, orange, pineapple,
and white grape juice concentrates even after 12 weeks of storage at
-23 degrees C (Oyarzabal et al., 2003). Yeast, mold fungi, lactic-acid
bacteria, and cocci log growth rate parallels deterioration of fruit
juice represented by spore mesophyll aerobes of the subtilis-mesentericus
type (Slovachevskaia et al., 1988). Pasteurization of apple cider is
therefore a validated treatment for ensuring adequate destruction of
E. coli 0157:H7, Salmonella spp., and L. monocytogenes (Mak et al., 2001,
Teo et al., 2001).
Preventing microbial infection of whole fruit is unavoidable. In three
field studies, samples of unwashed apples (drops and picked), washed
apples, and freshly pressed cider were presumptively analyzed for total
coliforms, E. coli, and enterococci using qualitative and/or quantitative
methods. Drop apples were more likely than picked apples to be contaminated
with E. coli (26.7% vs. 0%) and enterococci (20% vs. 0%). Washing had
little effect on coliform populations and in one field study was associated
with increased numbers. Total coliform populations in cider ranged
from <1
CFU/ml to >738 most probable number/ml, depending on the enumeration
method used and the sample origin. E. coli was not recovered from washed
apples or cider, but enterococci were present on 13% of washed apple
samples. The qualitative coliform method successfully detected these
bacteria on apples and in cider. Based on its exclusively fecal origin,
good survival in apple cider, and association with drop apples, research
concludes that E. coli is the most useful organism for confirming apple
and cider sanitation (Lang et al., 1999).
Reducing either the regeneration rate of commensal microbe proliferation
as apples ripen or reducing the rate of ripening without toxically
affecting the nutrient profile of the fruit using Zeolite-coated Potassium
Permanganate
(Z-KMnO4) in an enclosed packet was examined. Potassium
Permanganate (KMnO4) is a mild antiseptic/astringent, with
antimicrobial properties
(Anderson 2003). One study quantified the inactivation of the endotoxin
derived from Escherichia coli 055:B5 by Potassium Permanganate (KMnO4)
used as an oxidant in drinking water treatment and was shown to inactivate
1.0 endotoxin units (EU)/mLh derived from Escherichia coli 055:B5 (Anderson
et al., 2003). It was reported that potassium permanganate inactivated
90–100% of Pasteurella multocida strains (Karaivanov 1976, Brown
et al., 1978). The Salmonaella enzyme was observed to be oxidized/inactivated
by exposure to Potassium Permanganate (Roberts et al., 1975). Even
the highly resistant virus of Creutzfeldt Jakob disease, exposed to
a preparation
of potassium permanganate, is either inactivated or inhibited (Uysal & Kaaden
1993).
In a preliminary investigation, I excised 2 Red Delicious apple sections
from the same apple and refrigerated them separately for 7 days. One
sample was exposed to Zeolite-Coated Potassium Permanganate (Z-KMnO4)
and the other sample was not exposed to Z-KMnO4. The Z-KMnO4 sample presented
no outward visible spoilage/deterioration effects while the non-exposed
sample displayed significant observable deterioration. What then was
the numerical antimicrobial effect from Z-KMnO4 on aerobic bacteria and
yeast/mold growth rate?
In the present investigation, I repeated the preliminary experiment,
but extended refrigerated storage from 7 to 28 consecutive days. Taken
from a parent apple specimen, one apple section was exposed to Z-KMnO4
but not the other. Aerobic bacteria growth rate in the Z-KMnO4 exposed
sample was significantly inhibited (by a factor of 1000 X (10,000 AB/ml
Z-KMnO4 sample to 10,000,000 AB/ml non-exposed sample). It was further
observed that Z-KMnO4 did not inhibit Yeast/Mold (YM) growth.
Both samples following 28 days refrigeration yielded no significant difference
in
YM count/ml (Z-KMN04 = 100,000/ml YM: non-exposed sample = 100,000/ml
YM).

Materials and Methods
Red Delicious Apples. Random selected
produce was purchased from a Supermarket, Safeway Food & Drug,
1616 Northwest Blvd., Spokane, Washington, 99205.
Aerobic Bacteria and Yeast/Mold Test System Kits. Biosan Laboritories
donated a SaniCheckAB and a SaniCheckYM Test System For Counting
Aerobic Bacteria and Yeast/Mold. Biosan Laboratories, Inc.,
1950 Tobsal Court,
Warren Michigan, 48091–1351.
Zeolite-coated Potassium Permanganate (Z-KMnO4) packets
were donated by WayChem INC, P.O. Box 1450, 1101 Main Street, Evanston,
Wyoming,
82931.

Design
A single Red Delicious apple was randomly selected from a dozen specimens.
The outside of the apple was washed thoroughly with detergent soap
then washed again with a 1% solution of hydrogen peroxide (H2O2)
to inactivate transient microbes from the exterior skin surfaces.
The parent apple was sectioned into 25-gram and 28-gram samples,
respectively. Sterile technique was employed with all utensils prior
to placing samples inside two enclosed containers, one with a 7.5-gram
packet of Z-KMnO4 and one without. Both containers
were simultaneously stored in opposite refrigerator produce drawers.
Each refrigerated
compartment retained a consistent 45° F at 40% humidity for this
28-day storage period. After 28-days, each slice was blended in a
distilled water 1:10 solution then further triturated to 1:1000 solution.
Each solution's microbial count was determined by application
of a SaniCheckAB and SaniCheckYM test pad to each sample. Each sample
was incubated at 25–30°C (77–86°F) for either
36 hours for aerobic bacteria count or 72 hours for Yeast/Mold
count, respectively.

Results
When the phenolic compound in each
apple sample was exposed to air, a
predictable MaillardReaction,
browning of the fruit occurred at a more rapid rate in the control
sample than in the VCPP sample (See
Table I) or pictured example from a Gala Apple sample. In isolated
refrigeration compartments, aerobic bacteria growth within an
enclosed Red Delicious apple sample was inversely associated
with proximal
effective exposure to a single 7.5-gram packet containing Z-KMnO4.
The aerobic bacteria count following 28-days refrigeration, as
determined by a SaniCheckAB Test, was 10,000/ml in the Z-KMnO4 enclosed
sample. However, the aerobic bacteria count in the other apple
sample not
exposed to Z-KMnO4 was 10,000,000/ml. The Yeast/Mold
count following 28 days refrigeration in these samples, as determined
by
a SaniCheckYM
Test, was 100,000/ml in both Z-KMnO4 & non-exposed samples,
respectively. Aerobic bacteria growth rate was inhibited by a
factor of 1000 in
an apple sample exposed to Z-KMnO4 as compared to
an apple section from the original parent apple not exposed.
Yeast/Mold growth rate
was numerically equal in Z-KMnO4 and non-exposed samples,
respectively.

Discussion
Whole fruit or fruit juice may be
the source of food-borne illnesses due to pre-harvest contamination
or consumer-neglect from too
long storage. Fresh apples and unpasteurized apple juice receive
little
to no antimicrobial treatment and, despite their health-promoting
image, may transmit or harbor dangerous contaminants. Acid fruit
juices below pH 4.6 were once deemed a minor health threat due
to their high acidity. Furthermore, refrigeration temperatures
(below
5ºC) were thought to resist pathogen growth, until the discovery
that Listeria monocytogenes can
grow in temperatures as low as 2ºC.
Juice spoilage typically occurs as a reflection of the indigenous
microflora, yeast, mold and/or lactic acid bacteria growth. Nonetheless,
the emergence of hitherto unsuspected food pathogens with acid resistance
combined with an increase in susceptible individuals, immunocompromised,
chronically ill, the very young and very elderly, has dramatically
changed this picture. Safety must always take precedent with strict
limits on production, harvest, transportation, storage, manufacture,
processing, labeling and distribution. These are incorporated into
Good Agricultural Practices (GAPs) and Good Manufacturing Practices
(GMPs) with Hazard Analysis and Critical Control Point (HACCP) procedures
being applied throughout the food chain. These will be emphasized
as appropriate. The National Food Processors Association (NFPA) has
considered several options including current Good Manufacturing Practice
(GMP) regulations. One of NFPA's officers wrote, "The
only means of assuring that juice did not contain potentially pathogenic
microorganisms was to include a microbial control step that has been
scientifically proven to be effective in providing a level of protection
equivalent to pasteurization in the process. Two percent of all juice
products are not pasteurized or otherwise treated. Illness attributable
to raw juice or juice includes imported frozen raw (unpasteurized)
Mamey puree (13 cases, typhoid fever—Salmonella), raw apple
juice in Canada (E. coli 0157:H7), raw orange juice in Australia
(435 cases—Salmonella), raw orange juice from Arizona (300
cases—Salmonella muenchen in 20 states) and raw apple juice
in Tulsa, Oklahoma (9 cases—E. coli 0157:H7). Only a microbial
kill step applied to harvested raw fruit and/or juice itself can
ensure that potentially pathogenic microorganisms are eliminated.
Sorting and washing of fruit should be standard practice in all Good
Manufacturing Practice operations for juice production but cannot
be relied upon to ensure the complete removal of pathogenic microorganisms.
While theoretically possible, achieving an appropriate level of protection
from pathogenic microorganisms without applying some inactivating
treatment to the juice seems technologically infeasible at this time.
Processing methods that may provide an equivalent kill step include
batch and continuous high-pressure processing systems, pulsed electric
fields, ultraviolet light, electron beam treatment, irradiation,
ultra filtration, or use of one or more of the preceding treatments
in combination with an anti-microbial compound" (Matthys
AW).
Potassium permanganate (KMnO4) is a potent antimicrobial
compound, which acts as an oxidizing agent directly reducing the
contaminated
environment of indigenous toxic substances and/or aerobic bacteria.
KMnO4 is so potent that it should not come in direct
contact with humans or food nutrients. The packet-package of 7.5
grams Z-KMnO4 permits
the oxidizing, antimicrobial effect of the compound without transition
or absorption into the adjacent foods stored within the same compartment.
The chemical equation suggested by which ZPCC reduces ethylene
gas and subsequently inhibits fruit ripening rate is through oxidization,
namely as:
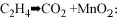
(Equation 1) 3CH2 + 2 KMnO4 + H2O = 2MnO2 + 3CH3CHO + 2 KOH
(Equation 2) 3CH3 CHO + 2 KMnO4+ H2O = 3CH3 COOH + 2MnO2 + 2KOH
(Equation 3) 3CH3 COOH + 8KMnO KMnO4 = 6CO2 +
8KMnO2 + 8KOH +
2H2O
(Equation 4) Combining equations 1–3 = 3CH2 CH2 + 12KMnO4
= 12MnO2 + 12KOH + 6CO2
Even if the reaction digresses or does not complete the carbon
dioxide-producing equation, many of the intermediate products
formed are irreversibly
bound to the media or act as a reactant. Such is the case of
the potassium hydroxide (KOH) formed in equations 1 and 2. The
KOH
will react with
the acetic acid formed in equation 2 to produce the potassium
acetate salt (KCOOCH3) through a simple acid-base neutralization
reaction
shown as:
(Equation 5) = CH3 COOH + KOH = KCOCH3 +
H2O, or:
(Equations 1,
2, and 5) = 3CH2CH2 + 4KMnO4 = 3KCOOCH3 +
4MnO2 + KOH =H2O

Conclusion
One 7.5-gram packet of Zeolite-Coated Potassium Permanganate sachet
stored in a plastic louvered container adjacent to an apple section
for 28-days was observed to remarkably reduce ripening rate and aerobic
bacteria growth by a factor of 1000, but failed to reduce the rate
of yeast/mold spore growth. The data observed presents an advantageous
method for reducing aerobic bacteria proliferation in
stored produce,
which is one marker of refrigerated spoilage, which otherwise
may compromise health or increase the risk of food-borne illness.
Z-KMnO4-reduced
produce ripening and subsequent aerobic bacteria contamination
by this intervention should not be employed as a panacea for
all indigenous
microbes, such as yeast/mold spores.
Aerobic Bacteria Count In Two Isolated 25–28
gram Apple Sections
Samples (Post-Refrigeration 28-Days)
| NO E.G.G. SAMPLE |
E.G.G. SAMPLE |
| 10,000,000/ml |
10,000/ml |
Visual View Two Isolated 25–28 gram Apple
Sections
(Post-Refrigeration 28-Days)
| Non-E.G.G. Sample |
E.G.G. Sample |
Extreme Maillard Reaction
Extreme Phenolic Browning |
Mild Maillard Reaction
Mild Phenolic Browning |
Is Ethylene Gas Control KMnO4 Safe?
E.G.G. pellets oxidize the ethylene gas with nascent oxygen (nascent
oxygen is a type of oxygen that oxidizes ethylene gas, molds, rots,
and odors), converting the pellets into manganese dioxide, which
is an organic fertilizer. Since February 2001, the Organic Material
Review Institute for use with Organic food production and the material
approve Ethylene Control E.G.G. for use and the FDA approves ink
that is made in the plastic EGG-content sachets.

Correspondence:
Bill Misner Ph.D.
West 1140 Glass Avenue
Spokane, Washington 99205
509-327-5817
800-336-1977
drbill@e-caps.com

Bibliography
Anderson I. Should potassium permanganate be used in wound care? Nurs
Times. 2003 Aug 5 11;99(31):61. [Abstract]
Anderson WB, Mayfield CI, Dixon DG, Huck PM. Endotoxin inactivation
by selected drinking water treatment oxidants. Water Res. 2003
Nov;37(19):4553–60.
[Abstract]
Bates R.P., Morris J.R., Crandall P.G. Principles and practices of
small—and medium—scale fruit juice processing, FAO
Agricultural Services Bulletin 146.
FAO 2001. [Table 4.1].
Blankenship S. Ethylene: The Ripening Hormone, 16th Annual Postharvest
Conference, Yakima, WA. March 14–15, 2000. Article # PC2000F.
[By permission, courtesy of the author, North Carolina State University,
Horticultural Science Department, Box 7609, Raleigh, NC 27695, [article
by sylvia_blankenship@ncsu.edu]
Brown P, Cathala F, Gajdusek DC. Creutzfeldt Jakob disease. Recommended
precautions for patient management and diagnostic procedures (author's
transl)] Rev Neurol (Paris).
1978 Apr;134(4):277–86. [Abstract]
Buta JG, Moline HE, Spaulding DW, Wang CY. Extending storage life of
fresh-cut apples using natural products and their derivatives. J
Agric Food Chem. 1999 Jan;47(1):1–6. [Abstract]
Han JH, Department of Food Science, Faculty of Agricultural & Food
Sciences, University of Manitoba, Winnipeg, MB, Canada—R3T 2N2
Tel: (204) 474–9295, by permission, courtesy of the author, Professor
J.H. Han. [Article]
Karaivanov L. Effect of physical and chemical factors on Pasteurella
multocida bacteriophages] Vet Med Nauki.
1976;13(4):65–72. [Abstract]
Lang MM, Ingham SC, Ingham BH. Verifying apple cider plant sanitation
and hazard analysis critical control point programs: choice of indicator
bacteria and testing methods. J Food Prot.
1999 Aug;62(8):887–93.
[Abstract]
Mak PP, Ingham BH, Ingham SC. Validation of apple cider pasteurization
treatments against Escherichia coli 0157:H7, Salmonella, and Listeria
monocytogenes. J Food Prot.
2001 Nov;64(11):1679–89. [Abstract]
Matthys AW, Vice President, Regulatory Affairs, National Food Processors
Association (Nfpa). "Comments on Juice Haccp," January
24, 2000. Re: Dockets Management Branch (HFA-305); Food and Drug Administration,
Docket No. 97N-0511; Hazard Analysis and Critical Control Point (HACCP);
Procedures for the Safe and Sanitary Processing and Importing of Juice;
Availability of New Data and Information and Reopening of Comment Period;
64 Federal Register 65669; November 23, 1999. [Article]
Mitcham EJ, Crisosto CH, Kader AA. Apple ‘Red Delicious' Ethylene
Control Recommendations for Maintaining Postharvest Quality. From:
Postharvest Technology Research and Information Center, Department
of Pomology, University of California One Shields Ave., Davis, CA 95616–8683.
Ethylene Control Inc., By permission, courtesy of Dave Biswell, President,
Ethylene Control Inc. July 2001. [Article]
Oyarzabal OA, Nogueira MC, Gombas DE. Survival of Escherichia coli
0157:H7, Listeria monocytogenes, and Salmonella in juice concentrates.
J Food Prot. 2003 Sep;66(9):1595–8. [Abstract]
Parish ME. Public health and nonpasteurized fruit juices. Crit
Rev Microbiol. 1997;23(2):109–19. Review. [Abstract]
Roberts MF, Switzer RL, Schubert KR. Inactivation of Salmonella phosphoribosylpyrophosphate
synthetase by oxidation of a specific sulfhydryl group with potassium
permanganate. J Biol Chem. 1975
Jul 25;250(14):5364–9. [Abstract/Full
JBC Text @: http://www.jbc.org/cgi/reprint/250/14/5364
Slovachevskaia EI, Stasiuk SN, Shenderovskaia LM. [Improved microbiological
control over the products of sublimation drying] Vopr Pitan.
1988 Nov-Dec;(6):60–2.
[Abstract]
Teo AY, Ravishankar S, Sizer CE. Effect of low-temperature, high-pressure
treatment on the survival of Escherichia coli 0157:H7 and Salmonella
in unpasteurized fruit juices. J Food Prot.
2001 Aug;64(8):1122–7.
[Abstract]
Uysal A, Kaaden OR. [Handling of unconventional pathogens] Pathologe.
1993 Dec;14(6):351–4. Review. [Abstract]

Acknowledgements
I wish to acknowledge Biosan Laboratories and WayChem Incorporated
for product donations to this research project:
Aerobic Bacteria and Yeast/Mold measures. Biosan Laboratories donated
a SaniCheckAB & SaniCheckYM Test System For Counting Aerobic Bacteria
and Yeast/Mold. Biosan Laboratories, Inc, 1950 Tobsal Court, Warren
Michigan, 48091–1351.
Zeolite-Coated Potassium Permanganate (Z-KMnO4) packets
were donated by WayChem INC, P.O. Box 1450, 1101 Main Street, Evanston,
Wyoming,
82931.
Disclosure: Neither competing interests nor remunerative relationship
exist between the author and Biosan Laboratories or WayChem Inc, respectively.
|




