|
Page 1, 2
A seemingly unfortunate thing happened while writing this column. After spending several hours on the project and saving the document to send for publication, lo and behold, I found that there was nothing there except my first rough draft. Despite time-consuming attempts to retrieve it, I am alas left to write it again. This has given me a second chance to reflect on what I deem important in women's health for the year 2012. There have been considerable policy and screening guideline changes and updates that have already affected and will continue to affect access to health-care services, screening, and preventive medicine, several of which I will review here. There has also been a robust amount of research in natural medicine in women's health, from simple dietary issues and bacterial vaginosis to more significant research that has settled some longstanding controversies regarding soy and breast cancer. So here goes, and while we have a plethora of medical information to absorb and contemplate and implement from 2012, we already can look to 2013 and more progress in the health of the American woman and research in women's health, including both conventional and alternative medicine.

The Affordable Care Act and Women's Health
The Affordable Care Act, enacted August 2012, helps to make preventive services affordable and accessible by requiring health insurance plans to cover preventive services recommended by the US Preventive Services Task Force (USPSTF) and an advisory committee on immunization and guidelines recommended by the Academy of Pediatrics. The act also requires insurance companies to cover select preventive health care for women and has adopted new guidelines that go a long way to improving some comprehensive services for women.
Well-woman visits: This would include an annual preventive care visit for adult women to obtain the recommended services, and additional visits if women and their health-care providers determine that they are necessary.
Gestational diabetes screening: This screening is for women 24 to 28 weeks' pregnant, and those at high risk of developing gestational diabetes.
HPV DNA testing: Women aged 30 or older will have access to high-risk human papillomavirus (HPV) DNA testing every three years, regardless of Pap smear results.
STI counseling: Sexually active women will have access to annual counseling on sexually transmitted infections (STIs).
HIV screening and counseling: Sexually active women will have access to annual counseling on HIV.
Contraception and contraceptive counseling: Women will have access to all Food and Drug Administration-approved contraceptive methods, sterilization procedures, and patient education and counseling. These recommendations do not include abortifacient drugs.
Breastfeeding support, supplies, and counseling: Pregnant and postpartum women will have access to comprehensive lactation support and counseling from trained providers, as well as breastfeeding equipment.
Interpersonal and domestic violence screening and counseling: Screening and counseling for interpersonal and domestic violence should be provided for all adolescent and adult women.
Anemia: screening on a routine basis for pregnant women
Bacteriuria: urinary tract or other infection screening for pregnant women
BRCA: counseling about genetic testing for women at higher risk
Breast Cancer Mammography: screenings every 1 to 2 years for women over 40
Breast Cancer Chemoprevention: counseling for women at higher risk
Chlamydia Infection: screening for younger women and other women at higher risk
Folic Acid: supplements for women who may become pregnant
Gonorrhea: screening for all women at higher risk
Hepatitis B: screening for pregnant women at their first prenatal visit
Syphilis: screening for all pregnant women or other women at increased risk
Osteoporosis: screening for women over age 60 depending on risk factors
Rh Incompatibility: screening for all pregnant women and follow-up testing for women at higher risk
Tobacco Use: screening and interventions for all women, and expanded counseling for pregnant tobacco users

Annual Visits/Exams
There has been a great deal of confusion and misinterpretation of the Pap smear screening guidelines and how they translate to exams and visits. Too often a woman goes to her practitioner's office and is told not come back for a Pap smear for 3 years or 5 years … this may be an appropriate recommendation according to the Pap smear guidelines (based on Pap smear history, risk of cervical dysplasia, age, and history of HPV) but not the same as annual well-woman gynecologic health assessments. The yearly visit is not dependent on receiving a Pap smear or not. The yearly visit can identify disease risks and current medical problems, optimize prevention strategies, discuss lifestyle issues (nutrition, exercise, smoking, alcohol, stress), and establish a good working relationship with your clinician.
I was glad to see that the American College of Obstetricians and Gynecologists (ACOG) recently updated its guidelines for well-woman gynecologic visits.
Major recommendations include:
- The first gynecologic visit should occur between age 13 and 15 and should emphasize education (body image, weight management, immunizations); I would add nutrition, exercise, avoidance of smoking and alcohol.
- Pelvic examinations are not necessary to test for sexually transmitted diseases or to initiate oral contraceptives in healthy, asymptomatic testing. Currently, even a urine sample can test for common STDs and a blood test can test for herpes simplex.
- Annual examinations of the external genitalia should be performed in all women; complete pelvic examination is recommended for all women 21 years and older. Internal examinations (visualizing the cervix and internally palpating the uterus and ovaries) can be made jointly by clinician and patient with discussion and information.
- Annual pelvic examination is not necessary. In healthy, asymptomatic women who have undergone a hysterectomy and removal of both ovaries, if that surgery has been done for noncancerous and nonprecancerous issues and the woman has no history of a genital tract neoplasia, or in women because of health status or age, I would choose not to act even if abnormalities were discovered.
- Clinical breast exams by the practitioner are recommended annually, despite a lack of clear evidence of benefit.
It is reassuring to know that ACOG has addressed this issue of well-woman gynecologic exams, as too many women, and even too many practitioners, end up not receiving/advising these visits other than when done on Pap smear intervals. It is important for all to realize that the "Pap smears" and "well-woman visits/pelvic exams" are not synonymous. Furthermore, the Affordable Care Act mandates insurance coverage of yearly well-woman visits.
Committee on gynecologic practice, the American College of Obstetricians and Gynecologists. Committee opinion no 534: Well-woman visit. Obstet Gynecol. 2012 Aug;12:421.

North American Menopause Society (NAMS) 2012 Position Statement
The position statement from NAMS on hormone therapy was last published in 2010. The updated statements further identify the benefit-risk ratio of estrogen therapy (ET) and combine estrogen-progestogen therapy (EPT). An advisory panel of experts is used to review the 2010 statement, evaluate new published evidence, reach a consensus, and then have it reviewed and approved by the NAMS board of directors. This document is an important read for conventional and alternative medicine practitioners in women's health. While it is a conservative group when it comes to compounded bioidentical hormones, a vital summary and review of the literature will greatly assist the clinician on the benefits and risks of hormone therapy in the areas of vasomotor symptoms, vaginal symptoms, quality of life, osteoporosis, cardiovascular events, coronary heart disease, stroke, venous thromboembolism, diabetes, endometrial cancer, breast cancer, ovarian cancer, lung cancer, mood, cognitive aging and dementia, and premature ovarian failure.
Here is a summary of the conclusions and recommendations for this 2012 position statement:
- Individualizing hormone therapy decision-making is key and should include her current health and quality-of-life priorities as well as her personal risk factors for the health issues mentioned above
- Duration of therapy is different for EPT than it is for ET. For EPT, the duration is influenced by the slight increased risk of breast cancer mortality associated with 3 to 5 years of use; for ET alone, there is a more favorable benefit-risk profile with at worst, a slight increased risk during an average of 7 years of use and 4 years of follow-up totaling a time period of 11 years.
- If local vulvovaginal symptoms are the only significant symptoms present, then local ET is recommended and ET is the most effective treatment of vulvovaginal atrophy.
- Women with premature ovarian failure or early menopause can use HT until age 51, the average age of menopause. This will help to prevent premature bone loss in particular. Longer treatment may be considered if needed to manage symptomatic issues
- There is a lack of data supporting the safety of using ET or EPT in breast cancer patients and use may in fact may increase breast cancer recurrence.
- Transdermal doses of estrogen and low doses of oral estrogen, when compared with standard oral doses, are associated with lower risks of venous thromboembolism and stroke.
The 2012 Hormone Therapy Position Statement of the North American Menopause Society. Menopause. 2012;19(3):257–271.

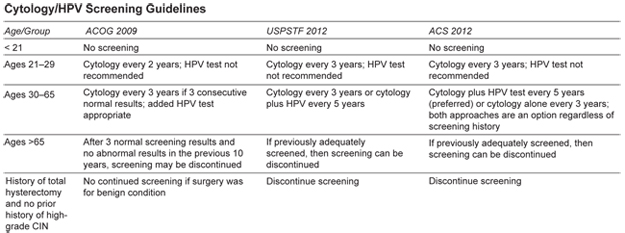
New Guidelines for Cervical Cancer Screening
Screening for cervical cancer with the historical Pap smear and in more recent years the liquid cytology technology has significantly lowered the incidence of cervical cancer. Over the last many years, medicine has an enhanced understanding of the natural history of the human papilloma virus (HPV) and has been able to conduct studies that compare screening with cytology alone compared with cytology plus HPV testing. Both the American Cancer Society and the USPSTF have updated their cervical cancer screening guidelines in 2012. These guidelines are intended to detect early abnormalities while reducing possible unnecessary colposcopy and biopsy procedures.
There are some additional recommendations in the new guidelines:
- None of the new recommendations apply to women who have immunosuppressive conditions such as: HIV positive, were exposed in utero to diethylstilbestrol, or had a prior treatment for CIN 2 or above.
- The recommendations do apply to women who have been vaccinated against HPV. Current HPV strains in the vaccinations do not include all pathogenic strains and included strains that account for only 70% of cervical cancers.
- HPV testing alone is not considered adequate screening
- Cervical screening algorithms results should be consulted for those women who test positive for HPV; they need closer follow-up than women who are HPV negative.
The downside of these new guidelines is that they tend to lead to fewer routine exam office visits for women. This then results in fewer breast exams, pelvic exams, blood pressure checks, contraception and family planning discussions, and assessments of lifestyle habits, mental health, and general health. While implementing these guidelines, we need to make it clear to patients that the reduced frequency of Pap smear testing does not mean reduced general exams and health screening.
Moyer V on behalf of USPSTF. Screening for cervical cancer: USPSTF recommendation statement. Ann Intern Med. Epub 2012 Mar 14.
Saslow D et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol. 2012 April;137:516.
Page 1, 2
|
![]()
![]()
![]()





